Introduction
Medicine is directly related to human life and therefore, its manufacturers have massive social responsibility of providing safe and effective medication. From its very initiation, Beximco Pharmaceuticals has always highlighted the need for rigid quality. Beximco’s commitment to quality is clear from its progressive use of state of the skilled manufacturing technology.
Beximco strictly follows WHO guidelines at every stage of manufacturing and procures its raw materials from the very best sources. But what makes Beximco a success story is its people. Beximco’s team of highly skilled and motivated professionals work around the clock to guarantee the safety and efficacy of its products.
Almost every professional subject demands proper practical knowledge in the own field. Thus industrial training program is one of the most important branch of a student studying in a professional subject like Pharmacy. Pharmacy is the art and science of preparing and dispensing medications and the provision of drug-related information to the community. It engross the explanation of prescription orders; the compounding, labeling, and dispensing of drugs and devices; drug product selection and drug utilization reviews; patient monitoring and interference; and the condition of cognitive services related to use of medications and devices. The American Pharmaceutical Association describes the mission of pharmacy as serving society as “the profession responsible for the appropriate use of medical cons, devices, and services to achieve optimal therapeutic outcomes.”
As per the syllabus of Bachelor of Pharmacy (Hons.) degree of Pharmacy Department every graduation student must endure in-plant training in a pharmaceutical industry. We are very lucky to get the opportunity of undergoing a training session under the auspices of BEXIMCO PHARMACEUTICALS LTD, internationally renowned leading pharmaceutical company in Bangladesh. Our training period taught us that only theoretical knowledge is not enough for proper understanding. We got our training in the Tongi Plant of Beximco Pharma. This Plant is really well equipped with up-to-the-minute technologies, which not only ensure the highest quality and precision of medicine but also contribute to the future development of medicine in Bangladesh.
We have gone through the books, read about the machinery, high tech process etc. of drug manufacturing We heard about the quality control, quality assurance etc. but when we were in Beximco Pharmaceutical Ltd. we found that only bookish knowledge is not enough to be a good pharmacist. Formerly we had only theoretical knowledge but now after the completion of four weeks in-plant training in BEXIMCO we have attained enough experience which would help us to make a career as a pharmacist with practical capability. In Beximco Pharmaceutical Ltd. we have seen more than what we had learnt.
Company Profile
History
Beximco Pharmacuticals Limited (BPL) is a leading edge pharmaceutical company and is a member of the BEXIMCO Group, the largest private sector industrial conglomerate in Bangladesh. BPLs strategic strengths include strong recognition of brands, highly skilled work force and diversified business mix. BPLs two brands – Neoceptin R (Ranitidine) and Napa (Paracetamol) are the top two selling brands in the Bangladesh Pharmaceutical market.
BPL started its operation in 1980, manufacturing products under the licenses of Bayer AG of Germany and Upjohn Inc. of USA and now have grown to become nation’s one of the leading pharmaceutical companies, supplying more than 10% of country’s total medicine need. Today Beximco Pharma manufactures and markets its own `branded generics’ for almost all diseases from AIDS to cancer, from infection to asthma, from hypertension to diabetes for both national and international markets.
BPL manufacture a range of dosage forms including tablets, capsules, dry syrup, powder, cream, ointment, suppositories, large volume intravenous fluids, metered dose inhalers etc. in several world-class manufacturing plants, ensuring high quality standards complying with the World Health Organization (WHO) approved current Good Manufacturing Practices (cGMP). BPL also contract manufacture for major international brands of leading multinational companies.
The recipient of three times `gold’ national export trophy, Beximco Pharma is the largest exporter of pharmaceuticals from Bangladesh. BPL is the only pharmaceutical company in Bangladesh received this highest national accolade for export, for record three times. BPL markets its brands through its own professional sales and marketing teams in African, South Asian and other markets. It also supply products to renowned hospitals and institutions in many countries, including Raffles Hospital and K K Women & Childrens’ Hospital in Singapore, MEDS and Kenyatta National Hospital (KNH) in Kenya, Jinnah Hospital, Agha Khan Hospital and Shaukat Khanum Memorial Hospital in Pakistan. BPL is also an enlisted supplier of World Health Organization (WHO) and UNICEF.
BPL has a strong market focus and is anticipating continued future growth by leveraging business capabilities and developing superior product brands and markets. In particular BPL is very interested in developing a strong export market in USA and Europe. To meet the future demand BPL has invested over US 50 million dollar to build a new state-of-the-art manufacturing plant, confirming to USFDA and UK MHRA standards. This new plant will also offer contract-manufacturing facility to leading pharmaceutical companies, especially from Europe and US.
BPL has employed over 2,300 staff and has an annual turnover of US 50 million dollar.
Key Information
| Year of Establishment | 1976 | |||||||||
| Country of Incorporation | Bangladesh | |||||||||
| Commercial Production | 1980 | |||||||||
| Status | Public Limited Company | |||||||||
| Business Lines | Manufacturing and marketing of pharmaceutical Finished Formulation Products, Large Volume Parenterals and Active Pharmaceutical Ingredients (APIs) | |||||||||
| Main Country of operation | Bangladesh | |||||||||
| Corporate Headquarter & Registered Address | 17 Dhanmondi R/A, Road No. 2, Dhaka- 1205, Bangladesh Phone : +880-2-8611891 (5 lines) Fax : +880-2-8613470 Email : beximchq@bol-online.com Website : www.beximco.net | |||||||||
| Operational Headquarter | 19 Dhanmondi R/A, Road No. 7, Dhaka- 1205, Bangladesh Phone : +880-2-8619151 (5 lines), +880-2-8619091 (5 lines) Fax : +880-2-8613888 Email : info@bpl.net Website : www.beximcopharma.com | |||||||||
| Overseas Offices & Associates | UK, USA, Pakistan, Myanmar, Singapore, Kenya, Yemen, Nepal, Vietnam, Cambodia and Sri Lanka | |||||||||
| Authorized Capital (Taka) | 2,000 million | |||||||||
| Paid-up Capital (Taka) | 1,040.97 million | |||||||||
| Number of Shareholders | Around 49,000 | |||||||||
| Stock Exchange Listings | Dhaka and Chittagong Stock Exchanges of Bangladesh and AIM of London Stock Exchange | |||||||||
| Number of Employees | 2,043 | |||||||||
| TIDM: (Tradable Instrument Display Mnemonic) | BXP | |||||||||
| Date shares were admitted to trading |
| |||||||||
| ISIN | US0885792061 |
Milestones
| 1976 | Registration of the company |
| 1980 | Started manufacturing and marketing of licensee products of Bayer AG of Germany and Upjohn Inc. of USA |
| 1983 | Launching of Beximco Pharma’s own brands |
| 1985 | Listing in the Dhaka Stock Exchange (DSE) as a Public Limited Company (PLC) |
| 1990 | Commissioning of Basic Chemical (APIs) unit |
| 1992 | Started export operation with Active Pharmaceutical Ingredients (APIs) |
| 1993 | First export market operation with finished formulations |
| 1996 | Introduction of Sustained Release Dosage form |
| 1997 | Introduction of Suppository Dosage form; Commissioning of Metered Dose Inhaler (MDI) plant; Introduction of Metered Dose Nasal Spray |
| 1998 | First pharmaceutical company of the country achieving ‘National Export Trophy (Gold)’ for 1994-95 |
| 1999 | UNICEF approval of Beximco Pharma as an enlisted supplier |
| 2000 | Agreement to manufacture Metered Dose Inhaler (MDI) for GlaxoSmithKline |
| 2001 | Introduction of Small Volume Parenteral (SVP) products; establishment of Analgesic-Antiinflammatory bulk drug plant |
| 2002 | Won the first prize of ICAB National Awards 2000 for ‘Best Published Accounts and Reports’ in Non-Financial Sector Category The first Bangladeshi company to supply pharmaceuticals to Raffles Hospital- the most prestigious hospital in Singapore |
| 2003 | Received “National Export Trophy (Gold)” for consecutive 2 years (1998-99, 1999-2000) Won the Silver prize of ICAB National Awards 2003 for ‘Best Published Accounts and Reports’ in Non-Financial Sector Category Won a tender to supply Neoceptin R and Neofloxin to Raffles Hospital of Singapore for the whole year’s consumption Introduced Anti-HIV drugs for the first time in Bangladesh Diversification into Anti-Cancer therapeutic class |
| 2004 | Signed contract with Novartis to manufacture their liquid, cream, ointment and suppository products under “Toll Manufacturing” agreement |
| 2005 | Merger of Beximco Infusions Ltd. with Beximco Pharmaceuticals Ltd. Admission to Alternative Investment Market (AIM) of London Stock Exchange (LSE) |
| 2008 | Become the first Bangladeshi company to achieve GMP Clearance from TGA Australia. Also become the first Bangladeshi company to receive export approval to Gulf countries. Beximco Pharmaceuticals Ltd. Is the first Pharmceutical company from Bangladesh to enter into Latin America. |
Vision
Building a healthier tomorrow
Products
BPL manufacture a range of dosage forms including tablets, capsules, dry syrup, powder, cream, ointment, suppositories, large volume intravenous fluids, metered dose inhalers etc.
Facilities
BPLs manufacturing facilities are spread across a 20 acres site located in and around Dhaka, Bangladesh. They comprise of a number of purpose built plants, including the new Oral Solid Dosage (OSD) plant. The land and buildings are wholly owned by the company and approximately 13 of the 20 acres of land are currently in use. The site includes facilities for manufacturing intravenous fluids, liquids, creams, ointments, suppositories, metered dose inhalers, active pharmaceutical ingredients as well as the existing and new OSD plants for tablets and capsules, the research laboratory and a number of warehouses. The plant and machinery throughout the site has been designed, procured and installed by partners from Germany, Switzerland, Sweden, Italy and the United Kingdom, amongst others.
The site has its own utility infrastructure to ensure the adequate generation and distribution of pure water at all times. The current installed electrical capacity is 4 MW and this will be increased to 8 MW soon. There are also water purifying and liquid nitrogen generation facilities on site. The entire site is at least 10 meters above sea level and is approximately 250 kilometers from the nearest sea – the Bay of Bengal.
Products
BPL manufacture a range of dosage forms including tablets, capsules, dry syrup, powder, cream, ointment, suppositories, large volume intravenous fluids, metered dose inhalers etc.
New Oral Solid Dosage (OSD) Plant
Beximco Pharma has invested over US $ 50 million on this new Oral Solid Dosage (OSD) plant complying with guidelines of highly regulated markets (for example, USFDA, UKMHRA, TGA Australia etc.). This state-of-the-art facility incorporates modern technological advancements.
The building finishes have been made to comply with BPLs design standards which have been prepared with the global standards (e.g. non shedding materials, resistant to cleaning agents, flush fitting etc.). Same is the case for HVAC system which has been installed to meet the global standards for air classification, air changes etc.
The manufacturing and packaging facilities have been designed to minimize generation and maximize containment of dust particles using closed transfer system and clean in place facility. All practicable measures have been taken to ensure that members of the staff are not exposed to unacceptable concentrations of dust particles. Process area, cubicles, storage area have been connected to vacuum dust cleaning. The design of the plant ensures automated materials handling systems and multilevel designs to enable gravity feed between processing stages. The building design has also allowed maximum engineering maintenance access without entering into the production areas.
Precise selection of equipment is also critical to improve process capability and ensure process robustness. A wide range of equipment has been procured from world renowned companies like Glatt, Romaco, Noack, Korsch and Nicomac.
A state-of-the-art chemical laboratory and dedicated microbiological laboratory equipped with a wide range of equipment have been set up to facilitate the quality control activities. The major equipment include HPLCs, UV spectrophotometer, FTIR spectrophotometer, AA Spectrophotometer, Karl Fischer titrator etc.
This plant provides greater capacity and a strong platform to launch Beximco Pharma’s products into highly regulated developed markets including Europe and the USA.
State of the art MDI Plant
The Metered Dose Inhaler (MDI) manufacturing plant of Beximco Pharma is one of the finest facilities in the world to produce Ozone benign HFA based MDI products. The plant was designed and installed under the technical collaboration of Pamasol, Switzerland.
The facility has dedicated areas for dispensing, manufacturing, canister and valve cleaning, canister filling, quarantine storage area, spray testing, packaging, propellant storage and in process control. As the inhaler manufacturing operation requires stringent control of humidity and temperature, automatic monitoring equipment are in use to continuously record temperature and relative humidity in areas like dispensing room, suspension manufacturing room, filling room, quarantine room etc.
The manufacturing equipment like manufacturing vessel, filling machine, weight checker, spray checking have been procured from Pamasol, Switzerland, a world renowned equipment supplier for Inhaler product manufacturing. The state-of-the-art Quality Control laboratory has been equipped with equipment like HPLC, Karl Fischer Titrator, Andersen Cascade Impactor etc.
BPLs MDI facility is the only approved outsourced facility of GlaxoSmithKline for their Ventolin® inhaler. They are happy to make their production and technical facilities open to inspection by existing and potential customers.
BPLs MDI plant has been audited by many local and overseas auditors and was acclaimed by them.
Due to the potential risk to the environment posed by CFCs, the technology shift in MDI industry is currently towards using hydrofluoroalkane (HFA) as propellants. Considering this changing trend in pulmonary drug delivery technologies, the company has already developed the HFA based MDIs which inevitably requires very high level of expertise and sophisticated technology. We are among the very few companies in the world manufacturing CFC-free HFA MDIs.
Now with this state-of-the-art MDI facility, the company aims at obtaining marketing approvals from stringent regulatory authorities of the developed markets.
IV Fluid Manufacturing Plant
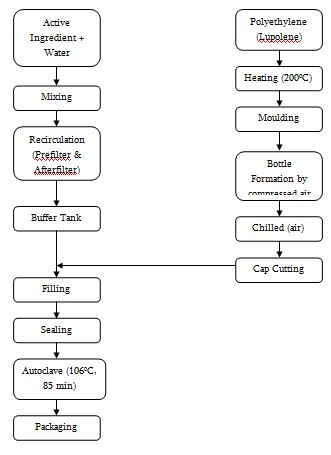
Beximco Pharma’s intravenous (IV) fluid manufacturing plant may be regarded as one of the most technologically advanced plants in the world. The plant was designed and installed in collaboration with Pharmaplan of Germany, a sister concern of Fresenius AG of Germany.
In designing the whole process, special care has been taken by providing absolute sterile manufacturing conditions. The prime feature of the process is that there is no human physical contact with the product at any given time. This has been ensured by way of a series of fully automated manufacturing procedures including robotics. The bottle pack aseptic system (Form-Fill-Seal or FFS) is a unique and innovative manufacturing technology. Plastic bottles are blow moulded, filled with the solution and sealed under sterile conditions, in a single working cycle where there is no environmental exposure or human contact during manufacturing. The IV fluids are presented in a scientifically designed bottle where there is an extra protective eurohead cap and a resealable rubber disk. The whole process is performed in a class 100 clean room. The air inside this room is cleaned upto 100 particles per cubic feet passing it through HEPA (High Efficiency Particulate Air) filters.
Thus, an advanced sterile environment is maintained in manufacturing the IV fluids in order to avoid the entry of bacteria, pyrogen and inert particles into products. This ensures the highest standards of quality and purity in order to ensure the highest level of safety.
The plant is ISO 9001: 2000 certified.
CHAPTER XII
Engineering Department
Major functions
The main aim of this dept is to support the production department for smooth running. Machineries of this department are of 2 types-
1. Production machinery
2. Utilities
A. Operation & maintenance of utility machineries are as follows:
a) Power supply
b) water system
c) Steam
d) Central compressor
e) Central air conditioning system
f) Dehumidification
g) Fork lift
h) Hand pallet truck
i) Vacuum cleaner
j) Gas
B. Maintenance of production machinery.
a) Scheduled maintenance
b) Troubleshooting or breakdown maintenance
C. Maintenance of Q.C machinery.
Utility Machineries
Power station
Machines Name Origin Capacity
Generator-1 WAUKESHA U.S.A 920 KW
Generator-2 CATERPILLAR U.S.A 1020 KW
Generator-3 WAUKESHA U.S.A 900 KW
Generator-4 WAUKESHA U.S.A 900 KW
Diesel generator DETROIT DIESEL ALLISON
Primary Requirements to run through Gas engine in the power station:
Gas
DM Water
Soft Water
Lube Oil
Battery
Compressed Air
Air
Boiler
There are 2 boiler machines.
Machines Name Origin Capacity
Boiler-1 Cleeve Brooks U.S.A. 3 Ton steam/hr
Boiler-2 Cleeve Brooks U.S.A. 2 Ton steam/hr
Hapa Foil Printer
Model: Hapa 226
Origin: Switzerlan
Water Purifying System
CHAPTER III
Human Resources Department & Safety
Major Functions
This department is related to the management of affairs. It concerns with the following:
HR recruitment planning, training & development, industrial relations, Performance appraisal, engineering, environmental safety & health etc. In a single word this department controls almost all the section in Beximco Pharmaceutical LTD.
- Recruitment of personnel with appropriate qualification and experience to fill all position that has an effect upon quality. Different standard are considered for different position.
- Assist new employees to complete joining activities and ensure placement of new comers.
- To arrange, induction-training program (orientation program) for new employees. After joining each employee is introduced with all of the departments and their functions to know about company operation.
- Prepare and Co-ordinate internship program for the students of different universities.
- Prepare Human Resources Inventory Report of the plant monthly. It includes director to daily labor of the company.
- Maintain & update personal files of all employees monthly. Such as Conformation of job, increment, promotion, transfer and other letters is adjusted in the employee file.
- Supervise and monitor employee attendance, job cards, prepare monthly attendance summary and daily absent report etc.
- Monitor and update leaves of plant employees. Each employee has a leave file which integrated the all kinds of leaves like
i. AL (Annual Leave)
ii. ML (Medical Leave)
iii. CL (Casual Leave)
iv. Special Leave
v. Maternity Leave
vi. Leave without pay etc
- Inform mangers and employees regarding, personnel polices and procedures of the company.
- Coordinate and monitor performance appraisal of plants employees annually.
- Assess the training needs of personnel in light with cGMP and other work related issues.
- Deal with industrial related issues like negotiation with employees union, ensure the labor rights etc.
- Ensure proper implementation of labor laws applicable to factory employees.
- Disciplinary action including suspension, punishment and termination.
- Ensure healthy labor management relationship for smooth production.
- Maintain liaison with Govt. regulatory Bodies. Human Resources department collaborate with following bodies for legal aid, help in any regulatory case.
- Ministry of industry
- Office of director of labor
- Explosive department
- Director of fire and Tongi fire station
- Tongi Thana, NSI, DB, SB.
- Custom & Vat, Tongi, circle
- DC office, Gazipur.
- SP office, Gazipur.
- Civil surgeon, Gazipur
- Ensure safety of all employees and company assets.
- Ensure proper security management of the plant.
- Handle external visitor and arrange necessary uniforms and other accessories.
- Supervise transport pool (Distribution, Repair, Maintance, etc).
- Supervise over all House Keeping and Gardening of the plant.
- Supervise washing facility to provide clean dress as per departmental daily requirement.
Safety
The safety department of Beximco Pharmaceuticals Limited functions along with the Human Resources Department. It usually provides the basic safety measurements to the employee.
- They provide first aid measures for chemicals in case of
Eye contact
Inhalation
Skin contact
Ingestion
2. They also give training to all employees about the activities in case of fire. Its the duty of all to make themselves familiar with
Position of the fire alarm button
Nearest escape from each room
Location of fire fighting equipments and their operating instruction
3. They also train up the employees how to escape from fire
If fire catches cloth
If there is a lot of smoke
Classes of fires | Definition | Fire extinguisher |
| Class A | Ordinary combustible materials such as wood ,paper | Water, chemical foam |
| Class B | Flammable liquid and gases such as kerosene and common organic solvents | Dry chemicals ,chemical foam |
| Class C | Electrical wires and equipments such as motor | Dry chemical |
| Class D | Combustible metals such Na, Mg etc | Sand |
CHAPTER XI
Infusion Unit: Large Volume Parenteral Formulation
Beximco Pharma’s intravenous (IV) fluid manufacturing plant may be regarded as one of the most technologically advanced plants in the world. The plant was designed and installed in collaboration with Pharmaplan of Germany, a sister concern of Fresenius AG of Germany.
In designing the whole process, special care has been taken by providing absolute sterile manufacturing conditions. The prime feature of the process is that there is no human physical contact with the product at any given time. This has been ensured by way of a series of fully automated manufacturing procedures including robotics. The bottle pack aseptic system (Form-Fill-Seal or FFS) is a unique and innovative manufacturing technology. Plastic bottles are blow moulded, filled with the solution and sealed under sterile conditions, in a single working cycle where there is no environmental exposure or human contact during manufacturing. The IV fluids are presented in a scientifically designed bottle where there is an extra protective eurohead cap and a resealable rubber disk. The whole process is performed in a class 100 clean room. The air inside this room is cleaned up to 100 particles per cubic feet passing it through HEPA (High Efficiency Particulate Air) filters.
Thus, an advanced sterile environment is maintained in manufacturing the IV fluids in order to avoid the entry of bacteria, pyrogen and inert particles into products. This ensures the highest standards of quality and purity in order to ensure the highest level of safety.
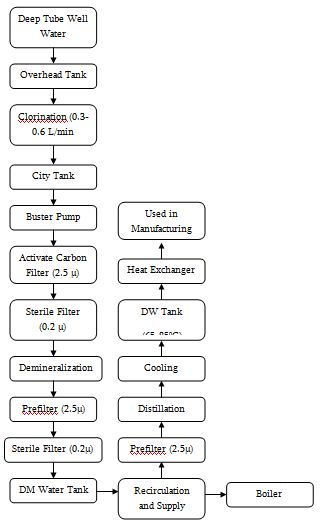
Water Treatment Process
Manufacturing and Packaging Steps
HVAC System
The HVAC system means Heating ventilation and Air conditioning system. The main objective of HVAC system is to maintain a constant temperature and a constant RH in the infusion production area, microbiology laboratory and the office rooms. It is the most modern concept of air conditioning which is the most special features in the infusion unit. HVAC is needed in Pharmaceuticals:
- To maintain specified temperature.
- To maintain specific relative humidity. (Less than 40% for some hygroscopic material like ranitidine).
- To remove dust particle from production area.
- To maintain proper airflow to the rooms ensuring that cross contamination does not occur.
- To prevent microbial contamination in some area by maintaining particle size within the tolerance range. (Using the HEPA Filters etc).
The process is maintained in the different area by controlling the air pressure (positive and negative).
Microbiology Laboratory
According to the ICH guidelines the microbiological test of raw materials and finished product is an important parameter of the quality drug. Accordingly the Infusion unit has a special microbiology laboratory in order to carry out the microbiological tests of several sensitive products.
The microbial test program includes the following:
- Test of raw material and packaging material.
- Test of finished product
- Environmental control
- Purified water test
- Monitoring of surfaces
- Personnel hygiene
The microbiology laboratory of contains the following separate rooms:
¨ Testing room & Incubation room:
¨ Media preparation room:
There is also a store room to store the reagents, cultures, standard microbes, chemicals and other testing instruments in a required condition
The total testing procedure can be summarized as follows:
Sample collection
Media preparation
Culture preparation
Incubation
Colony count
Identification
Final result
1. Test of Raw and Packaging Materials
Microbial limit test is performed which include:
1)Total aerobic viable count
2)Total aerobic bacterial count
3)Total fungal count
4)Presence or absence of Pathogens (E.coli, Salmonella sp. , P. aeruginosa, S. aureus)
Microbial Limit Test for Raw Materials and Finished Products (USP)
2. Test of Products
Bioburden check of intermediate products. It is done with the help of membrane filtration method. First 4 and last 4 bottles are tested for microbes.
Sterility test. 20 bottles of products are collected from critical points of the autoclave and are incubated for 14 days and observed.
Bacterial endotoxin test/ LAL test. It is an in-vitro test method for pyrogen. It has been developed utilizing the gelling property of the lysate of amoebocytes of limulus polyphemus. The flow diagram of LAL test is given below:
Sample
Specified ml of LAL, dissolve
Incubation at 370 C for 1hr
Gel formation
Indicate presence of endotoxin from gram-negative bacteria.
3. For the Environmental inspections following tests are performed
i) Settle Plate Method
ii) Air Sampler
4. Purified Water Test
It is done by membrane filtration method. Water is tested from 12 different points daily and 18 different points weekly.
5. Monitoring of surfaces
It is performed by:
i) Contact Plate Method
ii) Swap Test
6. Monitoring of personnel hygiene
Hands, gloves, appearances/clothes are tested by contact plate method.
List of Instrument of Infusion Unit
Sl. | Name of Instrument | Manufacturer/ Supplier | Origin | Uses |
1 | Deep Tube-well Aeration Chamber Sand Bed Filter | Sigma Eng. | Bangladesh | Raw water, supplied water for DM plant |
2 | Raw water reservoir (Overhead tank – 5000L) | Beximco Infusions Ltd. | ||
3 | Dosage station for chlorine with pump and container | LMI MILTON ROY | USA | Killing microorganisms |
4 | Filter housing for raw water | Pall | ||
5 | City water tank (2000L) | Beximco Infusions Ltd. | ||
6 | Booster pumps (3000L/H) | Grundfos | Germany | |
7 | Activated carbon filter (1100L) | Hager + Elsasser, GmbH | Germany | Replace organic substance from water and declorinate water for DM plant area |
8 | Filter house (2.5μ) | Pall | ||
9 | Filter house (0.22μ) | Pall | ||
10 | Demineralization plants (1 and 2) (Anion and cation exchanger) | Hager + Elsasser, GmbH | Germany | Production of DM water for distillation plants, boiler etc. |
11 | Mixed bed filter (3.5m3/h) | Hager + Elsasser, GmbH | Germany | Polishing DM waters |
12 | Filter housing (0.22μ) | Pall | ||
13 | Filter housing (0.22μ) | Pall | ||
14 | Regeneration and neutralization system (9000L) | Pharma Plan | Germany | |
15 | DM water tank (4870L) | Pharma Plan | Germany | |
16 | Distillation Plant 01 | EKSTROM & SONS | Sweden | |
17 | Distillation Plant 02 | KEMITERM ENGNEERING A/S | Sweden | Production of WFI and pure steam |
18 | Distillate Storage Tank 01 (7000 L) | Pharma Plan | Germany | |
19 | Distillate Storage Tank 02 (7500 L) | Getinge | Sweden | |
20 | Steam Boiler (2.6 T/H) | StandardKessel | Germany | Provide Technical steam |
21 | Oil Free Air Compressor 01 & 02 | Mehrer | Operating electronumeric valves & preparing sterile compressed air | |
22 | Water Chiller 01 | Finetee Century | Korea | Supply chilled water for HVAC & Production |
23 | Water Chiller 02 | Linde Aktiengesellschaft | Germany | Do |
24 | Water Chiller 03 | Kyungwon-Century Co. Ltd. | South Korea | Do |
25 | Air Handling Unit 01 & 02 | MEISSNER & WURST | Germany | Supply & maintain clean class, positive air pressure |
26 | Cooling Tower 01 | Linde AG, Mainz | Germany | Provide cooling air |
27 | Cooling Tower 02 | BSC | Singapore | Provide cooling air |
28 | Injection Moulding Machine | PO Yuen (To’s) Machine Factory | Hong Kong | Produce Cap |
29 | Preparation Tank 01 & 02 | Pharma Plan | Germany | |
30 | Preparation Tank 01 & 02 | Getinge | Sweden | |
31 | Fully Automatic Bottlepack Machine for 1000ml & 100ml Bottles | Rommelag | Germany | Bottlepacking |
32 | Cap Welding Machine for 1000ml & 100ml Bottles | Pharma Plan | Germany | Cap welding |
33 | Fully Automatic Bottlepack Machine for 500ml & 250ml Bottles | Rommelag | Germany | Bottlepacking |
34 | Cap Welding Machine for 500ml & 250ml Bottles | Pharma Plan | Germany | Cap Welding |
35 | Spray Type Autoclave | Sauter-Sulgen | Germany | Autoclaving |
36 | Spray Type Autoclave | Getinge | Sweden | Autoclaving |
37 | Pressing Conveyor Belt | Pharma Plan | Germany | Leak testing |
38 | Conveyor Belt | Pharma Plan | Germany | Conveying |
39 | Visual checking Devices | |||
40 | Conveyor Belt | Pharma Plan | Germany | Conveying |
41 | Labeling Machine with Batch No. Printer | Pharma Plan | Germany | Labeling |
42 | Ink Jet Printer | Image | France | Printing on Overpack |
CHAPTER VIII
Liquid Dosage Formulation
LCO Department of Beximco Pharmaceuticals Limited produces about 84 products of various dosages form including
(i) Syrup (example: Napa, Aristoplex)
(ii) Suspension (example: Lactameal, Flatameal DS)
(iii) Nasal spray (example: Decomit)
(iv) Drops (Example: Deflux Ped. Drop)
(v) Suppositories (example: Napa, Ultrafen)
(vi) Cream, Ointment, Gel (example: Neostin cream, Anustat ointment, Ultrafen gel)
LCO Department of BPL consist following 6 lines:
i. Line-1: Syrup, Suspension.
ii. Line-2: Antacid preparation
iii. Line-3: Small volume oral liquid preparations
iv. Line-4: Cream, Ointment
v. Line-5: Oral liquid suppository facility (OLSF), Syrup
vi. Line-6: Suppository
Observed Manufactured Products
Product Category | Product Name | Operations Observed |
| Syrup | Tofen | Filling, sealing & packing |
| Syrup | Napa (60ml & paediatric drop) | Manufacturing |
| Suspension | Flatameal DS | Filling, sealing & packing |
| Cream | Neostin | Manufacturing |
Machine list of Liquid, Cream, Ointment and Suppository Department,
Line #01
SL No. | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Manufacturing vessel | MV- 5000 | Tanners fabricated ltd, Australia. | 5000L | Manufacturing of liquid syrup & suspension. |
| 2. | Storage vessel & agitator | SV – 5000 | Tanners fabricated ltd, Australia | 5000L | Storage of liquid |
| 3. | Inline mixer & Transfer pump | IM – 600 | Silverson, England | 400L, 400psi | Online mixing & transfer of liquid syp & sus. |
| 4. | Filtering M/C millipore | FM-MP | Millipore, USA | 5000L/hr | Filtering of fluid |
| 5. | Vessel with Fluid agitator | FA – 942 | Lab. Equipment, India | 1000L | Stirring & mixing. |
Line #02
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Manufacturing vessel | MV- 3000 | Tanners fabricated ltd, Australia. | 3000L | Manufacturing of liquid suspension |
| 2. | Storage vessel & agitator | SV – 3000 | Tanners fabricated ltd, Australia. | 3000L | Storage of liquid suspension |
| 3. | Inline mixer | IM – 440 | Silverson, England | 600L , 440psi | Online mixing & transfer of liquid |
Line #03
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Fluid agitator | FA – 944 | Lab. Equipment, India | 250 rpm | Stirring & mixing |
| 2. | Liquid mixer Silverson | LM – SIL | Silverson, England | 450L | Liquid & cream homogenizer |
| 3. | Liquid transfer pump | LTP- 40 | Frymamachinen, Switzerland | 1000L/h | Transfer of liquid |
| 4. | Filtering M/C Sartorious | FA- SA | Sartorious, Germany | 1000L/h | Filter |
| 5. | Seitz Kettle | Germany | 200 L/h | Manufacturing vessele | |
| 6. | Vertical Ultrasonic cleaning machine | China | 50-200 pcs/min | Bottle washing | |
| 7. | FAR Infrared sterilizing and drying tunnel | Changsha, China | 50-300 pcs/min | Bottle drying | |
| 8. | Filling and Plugging machine | Changsha, China | 50-100 pcs/min | Bottle filling and sealing |
Line #04
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Planetary mixer | PLM-100 | Gansons, India | 50L | Mixing |
| 2. | Colloid mill | COLL-M | Probst & glass, Germany | 250 kg/hr | Mixing and blending of materials |
| 3. | General purpose mixing machine | GP-MM | Seitz, Germany | 100L | Mixing |
Line #05
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Manufacturing vessel | – | Tanners fabricated ltd, Australia. | 5000L | Manufacturing of liquid syrup & suspension |
| 2. | Storage vessel & agitator | – | Tanners fabricated ltd, Australia | 5000L | Storage of liquid |
| 3. | Filtering Machine millipore | – | Millipore, USA | 5000L/hr | Filtering of fluid |
| 4. | Auto Filling , Sealing, Bottle washing & Labeling machine
| – | Pharmalab, India | 6000Bot./hr | Washing, Filling, Sealing& Labeling of Bottles |
Line #06 (Suppository)
SL No | Machine Name | Model No. | Manufacturer | Capacity | Use |
| 1. | Sarong | D-402 | Italy | 5000 suppo/hr | Filling & Sealing of Suppository |
| 2. | Manufacturing vessel | – | Thailand | 100L | Manufacturing |
Unit: Filling
Line #01
SL No | Machine Name | Model No. | Manufacturer | Capacity | Use |
| 1. | Automatic liquid filling M/C(ATOMAT) | – | S.S Industries,India | 85-100b/m | 1.Bottle washing & drying 2. Liquid filling & cap sealing 3.Labelling & printing |
Line #02
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Automatic liquid filling M/C(ATOMAT) | Monobiock Filler | S.S Industries, India | 3000b/h | Liquid filling |
| 2.
| Cap sealing machine
| CS-V46 | Vaw, Germany | 28-32b/m | Cap sealing |
| 3. | Self adhesive labeling machine | – | Clear pack, Singapore | 110b/m | Bottle labeling |
Line #03
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Liquid filling machine | LFM-L1 | Local | 30-35b/m | Liquid filling |
| 2. | Cap sealing machine
| CS-V47 | Vaw, Germany | 28-32b/m | Cap sealing |
| 3. | Laminar Air Flow Cabinet | – | – | – | To control bacteria in the filling area |
Line #04
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Automatic tube filling & sealing machine
| 830920 | Greatide, Taiwan | 25-55 tube/min | Cream & Ointment filling & sealing |
Unit: Overprinting
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Semi-automatic over printing Machine | HAPA-L | Prontop hot hapa, Switzerland | 4000p/h | Printing label |
| 2. | Automatic over printing Machine | KK-610 | Jiangcozzer, Taiwan | 4000p/h | Printing label & carton |
| 3. | Semi-automatic printer | SAP-L | Local | 1500p/h | Printing label & carton |
| 4. | Automatic over printing machine | KK-560 | Taiwan | 4000p/h | Printing carton |
| 5.
| Automatic over printing machine | KK-550 | Taiwan | 4000p/h | Printing carton |
Bottle washing area
SL No | Machine Name | Model & Sr. No. | Manufacturer | Capacity | Use |
| 1. | Rotary bottle washer | DR W3 | Pharmalab, India | 5000b/h | Bottle washing |
| 2. | Rotary bottle dryer | STD | Gansons, India | 2500b/h | Bottle drying |
| 3. | Hot air dryer | – | Heraeus | 450b/load | Drying of (mainly pasteurization) bottle |
| 4. | Hot air dryer | HAD78 | Gansons, India | 830b/load | Drying of (mainly pasteurization) bottle |
| 5. | Sterilization oven Memmbrt | SO-MEM | MEMBRT, Germany | 800-840 b/load | Sterilization &drying |
| 6. | Rotary bottle washer | – | Pharmalab, India | 5000b/h | Bottle washing |
CHAPT-ER VI
Product Development Departmen
Product Development department deals with both new product formulation and reformulation of the existing products. BPL has the largest and well equipped Product Development Department ever in Bangladesh containing a group of competent and hard working personnel. The department’s concerned is to develop a better formulation to meet the patients growing need and to make it cost effective as well.
Functions of Product Development
(i) Launching of new products
(ii) Reformulation of existing products.
(iii) Reprocessing.
(iv) Market compliant handling.
(v) Troubleshooting.
(vi) Preparation of documents for export purpose.
Recipe
To develop a new drug the product development department has to collect DAR No. from Directorate of Drug Authority, Bangladesh after submission of recipe. A recipe contains:
Technical data
- Batch formula
- Manufacturing instructions
- Control data for active materials
- Control data for finished product
Pharmacological part
- Presentation
- Description
- Indications
- Contraindications
- Precautions
- Dosage and administration
- Adverse reactions
- Drug interactions
Reformulation
In case of existing products reformulation is performed when needed to improve the quality of the product or to minimize the cost of production to increase profit.
Annexure
Annexure is a document issued by the Drug Administration containing the
- DAR number
- Product name
- Pack size
- Composition
- Specification
- Overage
There are two types of annexure.
- Annexure I: For drugs except vitamins and antibiotics.
- Annexure II: For vitamins and antibiotics.
Trial Batch
Product Development Department manufactures trial batch for feasibility study. It also called Lab batch. It is the first batch of a product but it can’t be dispensed for market.
Pilot Batch
After approval of product, Product Development Department manufactures 3 batches of product for stability study. These 3 batches called Pilot batches.
Stability Study
Stability test is done for the following purposes.
To determine the expiry range
To determine the packaging mode
There are two methods of stability study
- Real time stability study
- Accelerated time stability study
Products of pilot batches preserved in three separated stability chambers of three different specific conditions.
These are
Real time stability
25° C , 60% Relative Humidity
- 30° C , 65% Relative Humidity
Accelerated time stability
40° C , 75% Relative Humidity
Conditions | Duration |
| 25° C , 60% RH | Up to Shelf life |
| 30° C , 65% RH | Up to Shelf life |
| 40° C , 75% RH | First 6 month |
If the degradation at accelerated stability study is less then 5% in 6 month, then the shelf life is 2 years.
Analytical Method Development
According to ICH guideline Analytical Method Development depends on the following parameters:
- Specificity
- Range
- Precision
- Linearity
- Accuracy
- Detection limit
- System stability testing
- Quantification limit
BMR & BPR processing
From the validation batches manufacturing, the validate procedures prepared as document record ship for each product. This is called Batch Manufacturing Record (BMR).
Batch Packaging Record is prepared for each product according to the product safety & cost.
List of Machines
Sl. | Name | Manufacturer/ Supplier | Origin | Uses |
1 | Compression machine (16 stations) | Manesty Machines Ltd. | England | Tablet compression |
2 | Coating machine | N.R. Industries Ltd. | Thailand | Tablet coating |
3 | Granulator | Pharmaceutical and Medical Supply Ltd. | Thailand | Granulation |
4 | Fluid bed dryer | Sapphire Machine Pvt. Ltd. | India | Drying |
5 | Multi-mill machine | Risesun Electrical & Industries Co. Ltd. | Milling | |
6 | Moister analyzer (Sartorius MA 30) | Imperial Corporation Ltd. | Germany | Determination of moisture content |
7 | Friability tester | Erweka | Germany | Determination of friability |
8 | Stirrer | Silverson | England | Mixing |
9 | Electronic balance | Shimadzu | Japan | Weighing |
10 | Dissolution tester (DT 600) | Erweka | Germany | Determination of dissolution |
11 | Disintegration tester | Disintegration testing | ||
12 | Sonicator | Ultrawave Ltd. | UK | Shaking |
13 | pH meter | Hanna Instrument | pH determination | |
14 | HPLC System 1 | Shimadzu | Japan | Identification, quantification, separation |
15 | HPLC System 2 | Shimadzu | Japan | Identification, quantification, separation |
CHAPTER IV
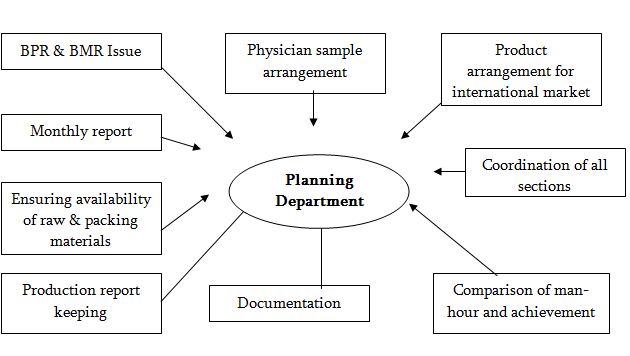
Production Planning Department
A well defined production plan is the pre-requisite for a well established & modern pharmaceutical industry, to achieve the following objectives-
To be a highly productive industry of good quality product.
To exist as an expert pharmaceutical industry in the world market.
A well-organized and modern pharmaceutical company like Beximco Pharmaceutical Ltd. always possesses a preplanned production schedule to ensure sound and uninterrupted production of medicine. BPL maintains a very well organized pragmatic production planning which is not only very easy to understand by the staffs but also very easy to apply to ensure the desired excellence of quality.
Its main functions are
Issue of B.P.R: According to the production plan B.P.R is issued by planning department. If any change required in B.P.R, planning will consult with product development for its correction.
Preparation of monthly plan & Its analysis: According to the demand of the market, planning department prepare a monthly plan and according to that plan they suggest production department to manufacture the product.
Ensure the availability of raw & Packing materials: Planning will arrange all types of raw & packing materials by consulting with head office for smooth production.
To Arrange physician sample: Production department will separate physician sample from bulk production according to the order of the planning department.
Arrange the products for international market: Planning will arrange & supply the products to the international market. After receiving information from E-mail, they take necessary action to meet that demand.
Co ordinate all sections for smooth production: Planning co-ordinate all sections to give highest production will in minimum cost and time.
Compare man hour & achievement: Planning compares man hour & achievement to know the actual efficiency of man and machine.
Keep daily production report & all documentation: Planning department keeps all types of daily production reports & Maintain major documentation.
Task of Production Planning Department at a glance
CHAPTER X
Quality Assurance Department
Quality assurance is a wide ranging concept covering all the matter which individually & collectively influences the quality of a product.
It is the sum total of all organized arrangement made with the objective of ensuring that medicinal products are of the quality required for their intended use.
Quality may be defined as level of excellence, which goes with the product. To assure this quality into the product is the main job of quality assurance department. QA department ensures that the quality is built in the product.
Quality (Definition according to DIN ISO 8402)
The totality of features and characteristics of a product or service that bear on its ability to satisfy stated or implied needs.
Quality Assurance (Definition according to DIN ISO 8402)
All planned and systematic actions necessary to provide adequate confidence that a product or service will satisfy given requirements for quality.
The impact of total quality maintenance is-
- Improved operating procedure.
- Greater customer satisfaction.
- Increased financial performance.
The following equipments are observed in The Solid QA station
| Machine | Manufacturer | Description/operation procedure |
| Hardness, Thickness, | Erweka, | It has 10 holes to hold 10 tablets to be |
| Diameter tester | Germany | tested. There are additional 2 holes, |
| one for measuring thickness, | ||
| diameter and Other for excretion | ||
| other tablet. | ||
| Electronic balance | Sartorius, | To perform weight variation of the |
| Germany | Tablet | |
| Disintegration tester | Erweka, | Six tablet holders and six plastic disks |
| Germany | for performing DT test. | |
| Friabilator | Erweka, | Perform friability tests at 25 rpm for |
| Germany | 4minutes. | |
| Heater | To maintain 37°c of the water bath. |
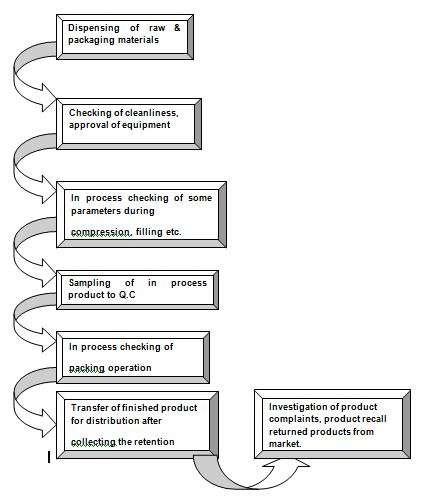
Flow chart for quality assurance activity
The functions of Q.A. in different section of the industry are given below
- Handling of new source sample.
- Artwork for packing material checking.
- Dispensing
A QA officer monitors dispensing process in the dispensing area and signs the dispensing card.
- Production area
In solid:
- i. Before granulation, the granulation area and the machinery are checked which are ready for granulation.
- ii. Before compression starts the machine and the rooms are checked to ensure proper cleaning.
- iii. During compression of tablets the following parameters are checked at regular interval-
Hardness, thickness, friability, weight-variation, disintegration, organoleptic tests for chewable tablets and other relevant parameter.
- iv. During capsule filling weight-variation & shell locking is tested.
- v. After compression of tablets or filling of capsules, the finished products are sent to QC department for analysis.
- vi. On the basis of the QC report &QA inspection of the tablets or capsules are approved for packing or rejected.
- vii. Tablets that require coating are sent to QC department for various tests before coating & after coating, the finished products are monitored by QA officers.
- viii. Before packaging starts the machine and the room conditions (temperature and humidity) are checked. During packing process the following parameters are checked:
Product strength, batch number, manufacturing date, DAR no, exp date, MRP, license no. foil printing, pocket formation, sealing, cutting, slitting, carton (inner and shipping), leaflet etc & checking of visual appearance of finished products.
- ix. Leak tests of plastic container, glass bottles, blisters and strips. are also performed.
In liquid
- i. The manufacturing vessels and area are checked before start manufacturing.
- ii. During manufacturing weight of ingredients, mixing time, temperature and rpm etc are checked.
- iii. Total volume is checked.
- iv. During filling fill weight per bottle is checked.
- v. Before packaging starts the machine and the room conditions (temperature and humidity) are checked.
- vi. Labels on bottles and on shipping cartons are checked.
In Suppository section
- i. Before start up of manufacturing or filling machines/rooms are checked for proper cleaning and good house keeping.
- ii. During manufacturing room temperature, vessel temperature, pump temperature, congealing temperature, room humidity, hardness, weight etc. are checked.
In Inhalation section
- i. The manufacturing vessel and area are checked properly before start up of manufacturing.
- ii. During manufacturing weight of the ingredients, mixing time, temperature, rpm etc. are checked.
- iii. During packaging the containers are checked for leak, total actuation no., dose/actuation, batch printing etc.
- iv. Sampling and approval/disapproval is done like other section.
Documentation
All kinds of documents related to production and control procedure are collected and preserved in QA department in an easily retrievable way. Some examples of the documents preserved in the QA department can be given below:
- Batch production record of a particular batch
- Raw material analytical sheet
- Packaging material analytical sheet
- Certificate of analysis of raw material
- Standard operating procedure (SOP)
- Material receiving report (MRR) sheet.
Retention control room
To force or encounter product complain for any batch product retention sample for every batch is preserved by the QA department. Specific amount of samples are collected from the packing line and they are kept in the retention sample room as a representative of that particular batch for a year excess of their claimed shelf life. All samples are kept in the conditions indicated in their label. If any complaints come from the market, the retention samples from that particular batch are collected from the retention sample room to verify.
CHAPTER IX
Quality Control Department
“Keep the quality up” with that slogan Quality Control Department of BEXIMCO PHARMA performs its day-to-day duties. Quality control is responsible for the day-by-day control of quality within the company. This department is stuffed with scientist and technicians who asses and assure that entire production process has been completed satisfactorily and satisfied all the aspects of GMP through preserving the purity and quality of the product.
Diagrammatic representation of present Q.C. activitiesActivities of Quality Control Department
- Receiving of the samples to be tested from QA department.
- Issuing release, reject or quarantine advice for each batch of raw material and final product.
- Assessment of the intermediate products and bulk products for further processing.
- Performing all test procedure for all incoming samples according to the schedule.
- Maintaining batch wise full quality control tests record and signature of the persons who perform the test.
- Performing environmental monitoring tests.
- Calibrations and standardization of laboratory equipments. 8. Ensuring precision and accuracy of all testing methods.
- Control of all laboratory reagents.
- Research and development of any new method and its validation.
- Testing of any return goods.
- Stability tests for finished products.
- Performing long time stability testing.
Good Manufacturing Practice (GMP)
GMP provides basic standards for the manufacturers of drugs and from the basis on which each company build its own systems procedures to assure the product quality. GMP ensures that products consistently produced and controlled to the quality standards appropriate to their intended use.
There are number of national & international GMP regulatory boards:
- WHO
- PIC
- FDA
- MHRA
- TGA
GLP norms and regulations
To ensure a wide range of testing disciplines the laboratory has some facilities which are discussed in GLP. The major considerations that accounts for GLP functioning involves:
1. Organization & management:
It is a basic requirement for establishing an effective QC system.
QC department is a well organized department. It has the authority of approval or rejection of each batch of starting, packaging and final materials on the basis of testing.
Personnel at all levels have accountability to carry out their responsibilities to meet the quality goal.
2. Personnel:
Qualified personnel are a key factor in ensuring quality. Personnel have three important characteristics:
Education.
Experience.
Training.
3. Premises:
A well designed premise is an essential part of GLP. It has:
Adequate space allocations
Easy to clean
Safety facilities
Storage facilities
Segregation of activities
Proper environment for testing
Every equipment has safety distance from others
Microbiology lab is isolated from Analytical lab
4. Equipments:
The laboratory is well equipped for performing all tests according to BP, USP. To maintain all equipments & machineries following measures are taken:
Every equipment has a log book for records
Regular maintenance is done.
All equipments are calibrated at specified intervals.
Most of the equipments & machineries are connected with printers thus there remain no chance of manipulation.
5. Reference standards:
Reference standards are substances with known purity or potency. Certified reference standards are available from many official sources. For routine laboratory tests it is worth considering working standards prepared by standardizing some good quality row materials against certified reference substance. A good stock of reference standards is maintained and used.
6. Reagents:
To maintain reagents QC department has taken following initiatives:
Laboratory has a complete list of all the reagents needed.
Solid reagents are stored in alphabetic orders and separated from liquid reagents.
The reagents need to be stored at low temperature is refrigerated.
Flammable reagents have segregated area.
Moisture sensitive reagents are stored in desiccants.
7. Procedure:
For every operation in QC written instructions are available. It has master control procedure (MCP) for every material. It has SOP for every action. SOP is strictly followed by analysts.
8. Sampling:
Appropriate sampling is a vital step in GLP. To facilitate correct and appropriate sampling procedures sampling must be done at conditions same as production environment e.g. sterile raw materials & products are sampled under laminar air flow.
9. Testing:
The incoming goods and finished products are tested according to BP, USP, EP as per requirement laid down specifications.
10. Documentation:
All test results are recorded on a test record sheet (TRS). All Q.C. records relating to a batch analysis are a part of the batch documentation. The purpose of documentation is to record important information with evidence. It is preserved at least one year after batch expired date.
Control of Raw Materials
Following characteristics are checked in raw materials.
- Description (Melting point, boiling point)
- IR
- Solubility
- LOD%
- Ash
- Impurities
Control of Packaging materials
Following characteristics are checked in packaging materials:
- Text of the printed materials.
- Color of the materials.
- Locking of cartoons.
- Compatibility of closures with bottles.
- Thickness of foils.
- Pasting of cartoons.
- Weight (gram per m2) of cartoons for checking materials.
- Transmittance test for glass bottle
- PVC-PVDC differentiation test
Control of finished products:
Final testing of finished product is made in the quality control laboratories. The testing of finished product for compliance with predetermined standards is a critical factor for product release. Following characteristics are checked in finished products.
- Description
- Identification
- DT and Dissolution
- Uniformity of weight
- Uniformity of content
Criteria of Validation
(i) System suitability
(ii) Accuracy
(iii) Precision
(iv) Linearity
(v) Specificity
(vi) Robustness & rigidness
(vii) Limit of detection
(viii) Limit of quantification
(ix) Range
Process validation:
Establishing documented evidence that provides a high degree of assurance that a specific process will consistently produce a product meeting its predefined specification & quality attributes. Good validation practice requires the close collaboration of departments such as those concerned with development, production, engineering quality assurance & control.
An adequate validation may be beneficial for the manufacturing in many ways-
- It deepens the understanding of process, decrease the risk of processing problem & thus assure the smooth running of the process.
- It decreases the risk of defect cost.
- It decreases the risk of regulatory non-compliance.
- A fully validated process may require less in process control & end product testing.
Sampling
Sampling may be defined as the process of removing an appropriate numbers of items from a population in order to make inferences to the entire population. The object of sampling and subsequent testing is to provide an effective check on the quality of the product or substances being processed.
In Beximco Pharmaceuticals Ltd, sampling is performed by the quality control department.
For the active ingredients, the sample is withdrawn from each container.
For the excipients, sampling is performed by the following formula- √n+1
Stability Study
Stability test is done for the following purposes.
For registration
Formulation changes
New drug development
Changes in primary package
To determine the expiry range
To determine the packaging mode
According to ICH Guidelines our country fall in zone III and IV. As a consequence the following conditions apply for the stability study.
| Condition | Storage condition | Testing frequency | |
Temperature | Relative Humidity | ||
| 1) Real Time | 25˚C ± 2˚C | 60% ± 5% | Initial-0,3,6,9,12 months 2nd-18,24 month 3rd-Annually |
| Or,30˚C±2˚C | 65% ± 5% | ||
| 2) Accelerated | 40˚C±2˚C | 75% ± 5% | 0,3,6 months |
In Beximco Pharmaceuticals Ltd. Real time & Accelerated condition is maintained. If significant changes occur in accelerated conditions then the product is observed for any significant changes in real time stability study for 1 year.
The significant changes in accelerated condition include
- Appearance of the product
- Dissolution for 12 minutes if not passed
- pH
- Impurities level
- Potency variation
QC Instrument List
Sl. | Name of Instrument | Model | Manufacturer | Origin | Uses |
1 | Electronic analytical precision balances | BP 210s | Sartorius AG | Germany | Analytical weighing |
2 | Electronic balance | PT 310 | Sartorius AG | Germany | Weighing |
3 | Electronic balance | EL 300 | Shimadzu | Japan | Do |
4 | Electronic balance (Top loading) | BD 1201 | Mettler Tledo AG | USA | Do |
5 | IR Moisture Analyser | MA 30 | Sartorius AG | Germany | Moisture determination |
6 | Disintegration Test Apparatus | ZT 3/6 | Erweka, GmbH | Germany | Disintegration testing |
7 | Dissolution Tester | DT 6L | Erweka, GmbH | Germany | Determination of dissolution |
8 | Dissolution Tester | DT 700LH | Erweka, GmbH | Germany | Do |
9 | Dissolution Tester | DT 608HH | Erweka, GmbH | Germany | Do |
10 | Rotating bottle disintegration tester | None | Fabricated in BPL | Do | |
11 | Suppository disintegration tester | ST 30 | Erweka, GmbH | Germany | Disintegration testing of suppository |
12 | Suppository melting point apparatus | SSP | Erweka, GmbH | Germany | Determination of melting point of suppository |
13 | Suppository softening time tester | PM3 | Erweka, GmbH | Germany | Determination of softening time of suppository |
14 | pH meter | PHI 32 | Bechman Instrument Inc. | USA | Determination of pH |
15 | Oven | UM600 | Memmert | Germany | Drying |
16 | Oven (Dry heat sterilizer) | TV 30U | Memmert | Germany | Drying |
17 | Vacuum oven | Gallenkamp | UK | Drying | |
18 | Humidity control oven | NEC-234R | Newtronic Equipment 10 | India | Drying |
19 | Incubator | Kottermann | Germany | Incubation | |
20 | Jolting StanPF volumeter | STAV2003 | Karl-Kolb Scientific Supplies | Germany | Tapping |
21 | Electromantle water | EM0500/C | Electromantle | UK | |
22 | Melting point apparatus | 7B1667C | Gallencamp | England | Identification by measuring melting point |
23 | Waterbath | Gallencamp | UK | Controlled heating | |
24 | Aerosizer LD | Amherst Process Instrument | USA | Size determination | |
25 | Gas chromatography system | Shimadzu Corp. | Japan | Identification | |
26 | UV-Visible Spectrophotometer | UV-160A | Shimadzu Corp. | Japan | Identification |
27 | HPLC System No.1 | Waters | USA | Identification, quantification, separation | |
28 | HPLC System No. 2 | Waters | USA | Do | |
29 | HPLC System No. 3 | Waters | USA | Do | |
30 | HPLC System No.4 | Shimadzu | Japan | Do | |
31 | Waters Breeze HPLC system | Waters | USA | Do | |
32 | Potentiometer autotitrator | 702 SM | Metrohm Ltd. | Potentiometric titration for ions | |
33 | Karl-Fisher titrator | DL 18 | Mettler | Switzerland | Moisture determination |
34 | Steam sterilizer (Autoclave) | NI-109-3S | Naniwa Ikakgyo Co. | Japan | |
35 | Fume chamber | 7591/F | Kottermann | Germany | |
36 | Digital hygrometer | Testo-625 | Testo | Germany | |
37 | Hotspot furnace (Muffle furnace) | Tactical 308 | Gallenkamp | UK | Determination of ash content |
38 | Leica AR-600 automatic refractometer | AR-600 | Leica | USA | To determine refractive index |
39 | Centrifuge | BB3V | Jouan | France | Separation |
40 | Centrifuge | 1000S3A | Gallenkamp | England | Separation |
41 | Polarizing Microscope | BHSP | Olympus Optical Company Ltd. | Japan | Identification |
42 | Brookfield viscometer | LVDVII+RT | Brookfield | USA | Determine viscosity |
43 | Fourier trasformation Infra-red spectrophotometer | IRPrestige-21 | Shimadzu Corp. | Japan | Identification |
44 | UV-Vis Spectrophotometer UV-1700 Pharmasec | UV-1700 | Shimadzu Corp. | Japan | Wide range analytical test |
45 | Atomic absorption spectrophotometer | AI 1200-Flame-VG | Aurora Instrument Ltd. | Canada | To identify and measure minerals |
46 | pH meter (Desktop) | pH 302 | Hanna Instrument Private Ltd. | Singapore | pH determination |
47 | ARIUM 611D1 Water purifying equipment | Sartorius AG | Germany | Water purification | |
48 | Automatic polarimeter | AA10 | England | To determine optical rotation | |
49 | Melting point apparatus | B530 | Buchi Laboratoriums Technic AG | Switzerland | Identification |
50 | Horizontal shaker | Shaking |
CHAPTER VII
Solid Dosage Formulation
Solid Department of BPL comprises the maximum number of products and also covers the major portion of its sales. Its current number of products is 261 consisting 83 plain tablets, 131 coated tablets, 37 capsules and 8 powders for suspensions.
Lay out of the general production (GP) area:
Raw material ware house
Dispensing
Dispensed material store
Sieving (If applicable)
Manufacturing Area
Granulation Unit
Granulation is the most preliminary process in the solid manufacturing area. Granulation is the process in which powder particles of raw materials are made to adhere to form larger particles called granules
- To improve the flow of powdered materials by forming sphere like or regularly shaped aggregates
- To improve the compression characteristics of the mix (blend.)
- To prevent segregation of the constituents in the powder mix.
Two types of granulation processes are performed in this unit:
-Dry granulation
-Wet granulation
Granulation units perform the following steps of tablet formulation to form larger particles in order to facilitate compression.
Packaging Unit
Packing can be defined as an economical means of providing, presentation, protection, identification/information, containment, convenience, and compliance for a product during storage, carriage, display and use until such time as the product is used or administered. After compression of tablets and coating [if required] the tablets are packed either in blister pack or in the strip.
There are two packaging areas:
a) Primary packaging area
In the primary packaging area, 10 machines are involved for packing. Among them, 8 for blister pack & 2 for strip pack.
b) Secondary packaging area
Types of packaging:
Packaging of Solid dosage form is of two types:
1) Strip packaging:
Striping materials is:
a) ALU-ALU type
b) PVC-ALU-PVC type
2) Blister Packaging:
Blistering materials are of three types-
a) ALU-ALU type
b) ALU-PVC type
c) ALU-PVDC type
Different parts of a blister pack machine
Different parts of a blister pack machine and their functions are giver in the following table:
Parts of blister machine | Function |
| 1. Base grid | Draws the base material, which may be PVC, PVDC, Aluminum foil. |
| 2. Forming Station | Forms blister in the base material either by hydraulic pressure or by air pressure and temperature |
| 3. Hopper | Tablets are kept in the hopper |
| 4. Chute and Spiral Feeder | Regulate the vibration so that table can move and fall in the feeding channel |
| 5. Feeding Channel | Feed the blister with tablet |
| 6. Deduster | Collects dust by creating vacuum |
| 7. Scanner | Scan the empty blister |
| 8. Sealing station | Seal the blister pack |
| 9. Cooling station | Cool the blister pack |
| 10. Marking station | Marks the empty blister |
| 11. Code embosser | Emboss code number |
| 12. Slitting station | Slit the blister pack |
| 13. Web clump | Draws the blister |
| 14. Punching Station | Cut the blister and slit down |
| 15. Timer | Regulate the passage of blisters |
| 16. Detector | Detect the empty packet |
| 17. Rejecter | Rejects the empty blister packet |
| 18. Conveyer belt | Convey the pack for further processing. |
Steps of Blister packing
|
|
|
| ||||
|
| ||||
|
| ||||
|
Steps of Strip Packing
|
|
|
| ||||
|
|
List of Machines in Solid Department
Sl.
| Name of Instrument | Manufacturer/ Supplier | Origin | Capacity | ||||
Granulation Unit #1 | ||||||||
1 | Planetary Mixer | Gansons | India | 60 Kg | ||||
2 | Fluid Bed Dryer | Saphire | India | 60 Kg | ||||
3 | Multi Mill | Gansons | India | 100 Kg/h | ||||
4 | Vac-U-Max | Belleville | USA | 2000 Kg/h | ||||
Granulation Unit #2 | ||||||||
5 | Planetary Mixer | Gansons | India | 120 Kg | ||||
6 | Fluid Bed Dryer | Saphire | India | 120 Kg | ||||
7 | Multi Mill | Gansons | India | 100 Kg/h | ||||
8 | Vac-U-Max | Belleville | USA | 2000 Kg/h | ||||
Granulation Unit #3 | ||||||||
9 | High Speed Mixer Granulator (HSMG) | Thailand | 250 Kg | |||||
10 | Fluid Bed Dryer | Saphire | India | 150 Kg | ||||
11 | Fluid Bed Dryer | Saphire | India | 150 Kg | ||||
12 | Multi Mill | Mark | Bangladesh | |||||
13 | Vac-U-Max | Belleville | USA | 2000 Kg/h | ||||
14 | Lift Lever (Stacker) | Toyota Tuscho Corp. | Japan | 650 Kg | ||||
Granulation Unit #4 | ||||||||
15 | Solace Aero Dryer | Solace | India | 120 Kg | ||||
16 | High Speed Mixer Granulator (HSMG) | Thailand | 250 Kg | |||||
17 | Multi Mill | Gansons | India | 100 Kg/h | ||||
18 | Vac-U-Max | Belleville | USA | 2000 Kg/h | ||||
Compression Units | ||||||||
19 | 16 Station Rotary Press | Manesty | England | 10560-29760 TPH | ||||
20 | 35 Station Rotary Press (Double Channel) (BB4) | Manesty | England | 70000-210000 TPH | ||||
21 | 35 Station Rotary Press (Double Channel) (BB4) | Manesty | England | 70000-210000 TPH | ||||
22 | 35 Station Rotary Press (Double Channel) (BB4) | Manesty | England | 70000-210000 TPH | ||||
23 | Fette P3100 (55 Station) | Fette | Germany | Max. 594000 TPH | ||||
24 | Fette P1200 (30 Station) | Fette | Germany | Max. 216000 TPH | ||||
25 | SEJONG (45 Station) | Sejong | South Korea | Max. 380000 TPH | ||||
26 | Weighing Balances | Sartorius | Germany | |||||
Coating Units | ||||||||
27 | Accela Cota 150 | Manesty | England | 150 Kg | ||||
28 | Accela Cota 350 A | Manesty | England | 350 Kg | ||||
29 | Accela Cota 350 B | Manesty | England | 350 Kg | ||||
30 | Sejong Cota | Sejong Pharmatek Ltd. | South Korea | 450 kg | ||||
Packaging Area | ||||||||
31 | Blister Machine (PG) | Precision Gear | India | 66000 TPH (4 Track) | ||||
32 | Blister Machine (085) | Klockner Hansel | Germany | 50000 TPH (2 Track) | ||||
33 | Blister Machine (042) | Otto Hansel | Germany | |||||
34 | Blister Machine (043) | |||||||
35 | Blister Machine (074) | Klockner Hansel | Germany | 48000 TPH (2 Track) | ||||
36 | ELMAC PACK Blister Machine | ELMAC | India | 36000 TPH (usually) | ||||
37 | PAM-PAC Blister Machine | India | 120000 TPH | |||||
38 | HOONG-A Blister Machine | Korea | ||||||
39 | Strip Machine | Gansons | India | Max. 80/min | ||||
40 | Strip Machine | Hemson | ||||||
CHAPTER XIII
Training Department
Training plays a vital role in translating organization objectives and plans into desired results.
Objectives:
- I. To expose the participants to the new concepts pharmaceutical manufacturing.
- II. To expose participants knowledge.
- III. To improve job skill.
- IV. To change attitude.
- V. To make GMP as a culture.
Training procedure:
Training need analysis
Select “Resource Person”
Preparing Training Calendar
Conducting Training Session
Evaluation of Training ,Trainee
Documentation
Retraining
Training type
Classroom Training
Audio-visual Training
Interactive learning
On the job Training
Group Exercise
Training Applies to
All persons engaged in manufacturing directly and indirectly.
Manager
Officer
Workman
Training Topics:
For New Employees
Induction training is conducted for New Employees on
Basic GMP
Safety overview
On the job
SOP’s
For Existing Employees
Train the trainer’s
GMP
Safety
Utility System
General Self Development
On the job training
v SOP’s
CHAPTER V
WAREHOUSE
Warehouse is a part of pharmaceutical industry where raw materials, packaging materials are stored before production and finished products are stored after production maintaining the specifications properly. BPL has a large and organized warehouse maintained by a group of sincere and hard working personnels.
- Different units of warehouse
- BPL Warehouse
- BIL Ware-house
- Pharmatek Ware-house
- Areas of Ware-house
- Quarantine area: After receiving raw and packaging materials are kept here for QA
approval.
- Released area: After getting approval raw and packaging materials are kept here with great safety. Released area is generally the central area of ware-house.
- In process area: According to store requisition(SR)dispensed materials for
manufacturing are kept here.
- Rejected area: Raw material, packaging material and finished products which are
unable to get approval from QA are retained here with great safety .Usually this area is located at the corner of the warehouse.
- Finished product area:-Generally finished products are started here for delivery to I & I service.
- Special area:-It includes cool room, for heat sensitive and flammable materials.
Identification of products and raw or packaging material in Ware-house
- Raw materials and finished products are easily identified here with the help of an index which includes different code for different area also.
- Packaging materials in warehouse are kept or placed following Alphabetical order.
Activities of ware-house
Activities of ware-house can be divided into 2 parts.
- Routine Activities
2. Periodic Activities.
Product release
Dispensing areas of Warehouse
One dispensing officer always responsible for dispensing the raw materials to the production and packing materials to the packing areas. Following things must be checked by the dispensing officer.
1) Check that only approved (green tag) materials are brought to the dispensing area.
2) Check that dispensing area is completely free from others materials.
3) Check that cleaning is done with IPA and savlon solution.
4) Check that correct quantity and approved quality of materials are being dispensed as per requisition.
5) Check that materials come first are being dispensed first, to follow FIFO (First In First Out).
Documentation in Dispensing
Documentation includes,
a) Serial number
b) Product name and code
c) Product batch number
d) Requisition entry
e) Cleaning
f) Material exit
g) Remarks
h) Tags
i) Signature of authorized person etc.
Inventory Functions of Warehouse
Inventory
After reading MRR to QA department materials will be kept pending list in computer
After getting released from QA department product will be at Norma inventory
Stock will be deducted from stock against S.R.(Store requisition) after dispensing.
Conclusion
We are fortunate enough to get in-plant training opportunity in BPL covering a large number of dosage forms. Our four weeks observation in various departments helped us to make our theoretical knowledge clear. We have observed manufacturing procedures, quality control, packaging and other related activities of pharmaceutical products and fulfilled our thirst.
We have found a very friendly and cordial environment during our training periods. The personnel were cordial to make us known to various departmental activities and machines as well.
We have observed various latest technology applied valuable machines and large industrial area in BPL containing own power stations and liquid nitrogen plant. We have also observed strict quality assurance in every step before supplying the product to the national and international market. In this sense we are lucky and happy to see such practice of GMP.
At last we would like to give hearty thanks to BPL and our gratitude goes to its all personnel. We wish better cooperation between our Department and BPL day by day.
We wish BPL’s good luck to attain the desired goals in national and international market.