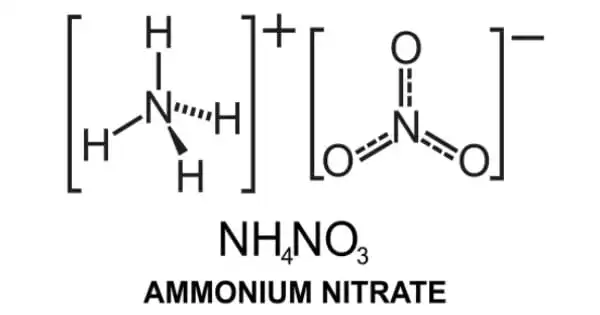
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline substance made up of ammonium and nitrate ions. Although it does not form hydrates, it is highly soluble in water and hygroscopic as a solid. It is mostly used as a high-nitrogen fertilizer in agriculture. In 2017, global production was predicted to be 21.6 million tonnes. It is commercially accessible in two forms: as a colorless crystalline solid and as prills for specific purposes.
Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, a popular industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices.
Properties
Ammonium nitrate is the ammonium salt of nitric acid. It has a role as a fertilizer, an explosive, and an oxidizing agent. It is an inorganic molecular entity, an ammonium salt, and an inorganic nitrate salt. It is an ionic salt made up of the ammonium cation (NH4)+ and the nitrate anion (NO3)–.
- Molar Mass/ Molecular Weight: 80.043 grams per mole
- Density: 1.725 grams per cubic centimeter
- Melting Point: 442.8K (169.6oC)
- Boiling Point: Decomposes at 483K (210oC)

Production, reactions, and crystalline phases
The industrial production of ammonium nitrate entails the acid-base reaction of ammonia with nitric acid:
HNO3 + NH3 → NH4NO3
Ammonia is used anhydrously (as a gas), and nitric acid is concentrated. Because of its extremely exothermic nature, the reaction is violent. After the solution is created, usually at around 83 percent concentration, the excess water is evaporated away, leaving an ammonium nitrate (AN) content ranging from 95 percent to 99.9 percent concentration (AN melt), depending on grade. In a spray tower, the AN melt is formed into “prills” or small beads, or into granules by spraying and tumbling in a revolving drum. To prevent caking, the prills or granules might be dried, chilled, and coated. These prills or granules are the most common AN products on the market.
The ammonia required for this process is obtained by the Haber process from nitrogen and hydrogen. Ammonia produced by the Haber process can be oxidized to nitric acid by the Ostwald process. Another production method is a variant of the nitrophosphate process:
Ca(NO3)2 + 2 NH3 + CO2 + H2O → 2 NH4NO3 + CaCO3
The products, calcium carbonate, and ammonium nitrate, may be separately purified or sold combined as calcium ammonium nitrate.
Ammonium nitrate can also be made via metathesis reactions:
(NH4)2SO4 + Ba(NO3)2 → 2 NH4NO3 + BaSO4
NH4Cl + AgNO3 → NH4NO3 + AgCl
Reactions
As ammonium nitrate is a salt, both the cation, NH4+, and the anion, NO3−, may take part in chemical reactions.
Solid ammonium nitrate decomposes on heating. At temperatures below around 300 °C, the decomposition mainly produces nitrous oxide and water:
NH4NO3 → N2O + 2H2O
At higher temperatures, the following reaction predominates.
2 NH4NO3 → 2N2 + O2 + 4H2O
Both decomposition reactions are exothermic and their products are gas. Under certain conditions, this can lead to a runaway reaction, with the decomposition process becoming explosive. See § Disasters for details. Many ammonium nitrate disasters, with loss of lives, have occurred.
The red–orange colour in an explosion cloud is due to nitrogen dioxide, a secondary reaction product.
Occurrence
Ammonium nitrate is found naturally as the mineral gwihabaite (formerly known as nitrammite) – the ammonium analogue of saltpetre (mineralogical name: niter) – in the driest regions of Chile’s Atacama Desert, often as a crust on the ground or in conjunction with another nitrate, iodate, and halide minerals. It was mined for ammonium nitrate there until the Haber–Bosch process made it possible to synthesis nitrates from atmospheric nitrogen, leaving nitrate mining obsolete.
Uses
Some important applications of this ionic salt are listed below.
- It is a key component of many fertilizers since it is quite rich in nitrogen (34%).
- When mixed with fuel oil, it forms an explosive known as ANFO, which is one of the most popular industrial explosives.
- It is also used in the mining and construction industry as a component of explosives.