Principle:
The Baeyer–Villiger oxidation is an organic reaction in which a ketone is oxidized to an ester by treatment with peroxy acids or hydrogen peroxide.Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry. It is named after the German chemist Johann Friedrich Wilhelm Adolf von Baeyer (1835–1917) and the Swiss chemist Victor Villiger (1868–1934).
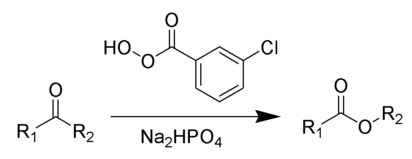
Reagents typically used to carry out this rearrangement are meta-chloroperoxybenzoic acid (mCPBA), Disodium phosphate or sodium bicarbonate is often added as a buffering agent to prevent transesterification or hydrolysis.
Mechanism
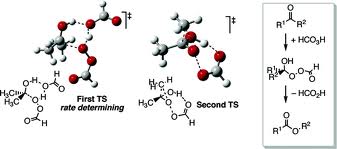
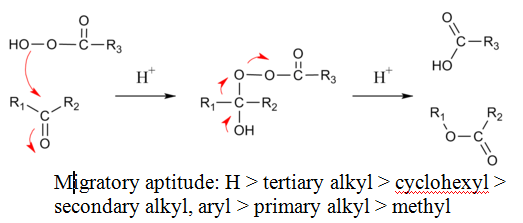
The reaction mechanism of this oxidative cleavage involves first addition of the peroxy acid to the carbonyl forming a tetrahedral intermediate also called the Criegee intermediate for its similarity with rearrangement of that name. The transition state for this step is envisioned as a hydrogen relay involving three peroxy acid molecules with linear O-H-O interactions.[7] Next is a concerted migration of one of the adjacent carbons to oxygen with loss of a carboxylic acid. If the migrating carbon is chiral, the stereochemistry is retained.
In the transition state for this migration step the R-C-O-O dihedral angle should be 180° in order to maximise the interaction between the filled R-C sigma bond and the antibonding O-O sigma bond. This step is also (at least in silico) assisted by two or three peroxyacid units enabling the hydroxyl proton to shuttle to its new position.
For unsymmetrical ketones, the migrating group is usually the one that can best stabilize positive charge. Thus, cyclic ketones produce lactones and aldehydes usually produce carboxylic acids, although formates can also be formed if the migrating group is tertiary or an electron rich vinyl group or aromatic ring (Dakin reaction). Sometimes the alcohol is formed when the formate is hydrolytically unstable.
This mechanism was first proposed by Doering and Dorfman in 1953 and based on isotope labeling experiments.
Application
- Air Pollution and Industrial Hygiene
- Apparatus and Plant Equipment
- Cement, Concrete, and Related Building Materials
- Ceramics
- Electrochemical, Radiational, and Thermal Energy Technology
- Essential Oils and Cosmetics
- Extractive Metallurgy
- Ferrous Metals and Alloys
- Fossil Fuels, Derivatives, and Related Products
- Industrial Inorganic Chemicals
- Pharmaceutical Analysis
- Pharmaceuticals
- Physical Organic Chemistry
- Steroids
- Terpenes and Terpenoids
- General Physical Chemistry
- Inorganic Analytical Chemistry
- Inorganic Chemicals and Reactions
Reference :
- ^ Baeyer, A.; Villiger, V. (1899). “Einwirkung des Caro’schen Reagens auf Ketone” (abstract). Ber. 32 (3): 3625–3633. doi:10.1002/cber.189903203151.
- ^ Baeyer, A.; Villiger, V. (1900). “Ueber die Einwirkung des Caro’schen Reagens auf Ketone” (abstract). Ber. 33 (1): 858–864. doi:10.1002/cber.190003301153.
- ^ Crudden, C. M.; Chen, A. C.; Calhoun, L. A. (2000). “A Demonstration of the Primary Stereoelectronic Effect in the Baeyer-Villiger Oxidation of α-Fluorocyclohexanones”. Angew. Chem. Int. Ed. 39 (16): 2851–2855. doi:10.1002/1521-3773(20000818)39:16<2851::AID-ANIE2851>3.0.CO;2-Y.
- ^ Krow, G. R. Org. React. 1993, 43, 251. (doi: 10.1002/0471264180.or043.03)
^ Burton, J.W.; Clark, J.S.; Derrer, S.; Stork, T.C.;