Every year, around one million people worldwide become infected with the HIV virus, which causes AIDS. To replicate and spread the infection, the virus must insert its genetic material into the cell nucleus and integrate it into a chromosome. Researchers led by Dirk Görlich of the Max Planck Institute for Multidisciplinary Science and Thomas Schwartz of the Massachusetts Institute of Technology (MIT) have discovered that its capsid has evolved into a molecular transporter. As a result, it can immediately penetrate a critical barrier that ordinarily shields the cell nucleus from viral invaders. This method of smuggling renders the viral DNA invisible to antiviral sensors in the cytoplasm.
Forty years after the human immunodeficiency virus (HIV) was identified as the cause of AIDS, we have treatments that effectively control the disease, but there is still no cure. The virus infects specific immune cells and uses their genetic program to grow and duplicate its own genetic material. The infected cells subsequently create the next generation of viruses, which are eventually eliminated. The immunodeficiency symptoms of AIDS are caused by the huge loss of immune cells that ordinarily fight viruses and other infections.
To utilize the host cell’s resources, HIV must smuggle its genetic material over cellular defense lines and into the cell nucleus. The nucleus, however, is jealously guarded. Its nuclear membrane keeps undesirable proteins and viruses out of the nucleus, as well as macromolecules from escaping uncontrollably. Nonetheless, certain proteins can pass since the barrier is not completely shut.
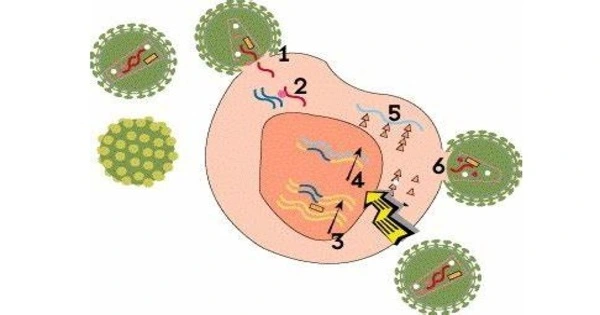
HIV packages its genome into a capsid. Recent evidence suggests that the genome stays inside the capsid until it reaches the nucleus, and thus also when passing the nuclear pore. But there is a size problem.
Thomas Schwartz
The nuclear envelope has thousands of small nuclear pores that serve as passageways. They govern these transport processes using importins and exportins, which are molecular transporters that catch payloads with valid molecular “passcodes” and convey them through the nuclear pore channel. A’smart’ substance transforms these pores into one of nature’s most efficient sorting and transport systems.
“Smart” sorting in the nuclear pore
This “smart” material, called FG phase, is jelly-like and impenetrable for most macromolecules. It fills and blocks the nuclear pore channel. Importins and exportins, however, can pass through because their surfaces are optimized for sliding through an FG phase.
The cell’s border control in the FG phase happens extremely fast — within milliseconds. Likewise, its transport capacity is enormous: a single nuclear pore can transfer up to 1,000 transporters per second through its channel. Even with such a high traffic density, the barrier of nuclear pores remains intact and keeps suppressing unwanted border crossings. HIV, however, subverts this control.
Smuggled genetic material
“HIV packages its genome into a capsid. Recent evidence suggests that the genome stays inside the capsid until it reaches the nucleus, and thus also when passing the nuclear pore. But there is a size problem,” Thomas Schwartz of MIT explains. The central pore channel is 40 to 60 nanometers wide. The capsid has a width of about 60 nanometers and could just squeeze through the pore. However, a normal cellular cargo would still be covered by a transporter layer that adds at least another ten nanometers. The HIV capsid would then be 70 nanometers wide — too big for a nuclear pore. “Nevertheless, cryo-electron tomography has shown that the HIV capsid gets into the nuclear pore. But how this happens has been so far a mystery in HIV infection,” says Max Planck Director Görlich.
Camouflage as a molecular transporter
He and Schwartz have now identified how the virus overcomes its size challenge, which is accomplished by clever molecular adaptations. “The HIV capsid evolved into a transporter with an importin-like surface. This allows it to pass through the FG phase of the nuclear pore. The HIV capsid can thus reach the nuclear pore without the assistance of transporters, bypassing the protective mechanism that normally stops viruses from accessing the cell nucleus,” the biochemist explains.
His team was successful in replicating FG stages in the laboratory. “Under the microscope, FG phases appear as micrometer-sized spheres that completely exclude normal proteins, but virtually suck up the HIV capsid with its enclosed contents,” explains Liran Fu, one of the study’s first authors, in Nature.
In one important way, the HIV capsid differs from previously characterized transporters that pass through nuclear pores: it entirely encapsulates its cargo, concealing its genetic payload from antiviral sensors in the cytoplasm. Using this method, viral genetic material can be carried past the cellular virus defense mechanism without being detected and killed. “This makes it another class of molecular transporters alongside importins and exportins,” goes on to say.
There are still many unanswered questions, such as where and how the capsid disintegrates to release its contents. However, the observation that the capsid is an importin-like transporter might one day be exploited for better AIDS therapies.