Calcium Hydroxide
Definition
Calcium hydroxide is also called slaked lime, Ca(OH)2, which is obtained by the action of water on calcium oxide. It is colorless crystal or white powder. When heated above 580°C it dehydrates, forming the oxide. Like the oxide, it has many uses, e.g., in liming soil, in sugar refining, and in preparing other compounds. It is a strong base and is widely used as an inexpensive alkali, often as a suspension in water (milk of lime); it is used in leather tanning to remove hair from hides.

It has many names including hydrated lime, caustic lime, builders’ lime, slack lime, cal, or pickling lime. Calcium hydroxide is used as an industrial alkali and as a constituent of mortars, plasters, and cement. It is used in the kraft paper process and as a flocculant in sewage treatment.
Structure and Properties of Calcium Hydroxide
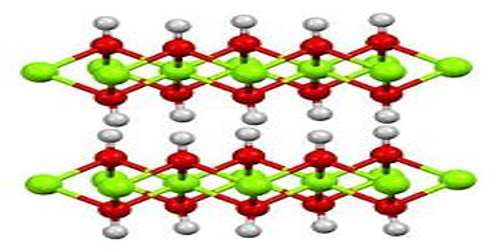
The process of producing calcium hydroxide can also easily be reversed by heating it to 512°C, at which point the water is driven off, reverting the compound to calcium oxide. Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH)2; i.e., the cadmium iodide motif. Strong hydrogen bonds exist between the layers.
In the laboratory it can be prepared by mixing aqueous solutions of calcium chloride and sodium hydroxide. The mineral form, portlandite, is relatively rare but can be found in some volcanic, plutonic, and metamorphic rocks. It has also been known to arise in burning coal dumps.
Calcium hydroxide is obtained as colorless crystals or a white powder with a density of 2.21 g/cm³ and melting point of 580 °C.
It is relatively soluble in water. It partially dissolves in water to produce a solution called limewater, which is a moderate base. Limewater or aq. Ca(OH)2 reacts with acids to form salts, and it can attack some metals such as aluminum. Limewater readily reacts with carbon dioxide to form calcium carbonate, a useful process called carbonatation:
Ca(OH)2 + CO2 → CaCO3 + H2O
Applications of Calcium Hydroxide
Calcium hydroxide has many uses, e.g., in liming soil, in sugar refining, and in preparing other compounds. It is a strong base and is widely used as an inexpensive alkali, often as a suspension in water (milk of lime); it is used in leather tanning to remove hair from hides. It is used in whitewash, mortar, and plaster. It is also an important ingredient in cement, plaster and mortars. As a fairly non-toxic and mild base, it has many uses in the food industry, including pH adjustment, calcium fortification, digestion aid, and baking soda substitute.

Limewater is a clear, saturated water solution of calcium hydroxide. It is used in medicine to treat acid burns and as an antacid. Because calcium hydroxide readily reacts with carbon dioxide, CO2, to form calcium carbonate, a mixture of gases can be tested for the presence of CO2 by shaking it with limewater in a clear container; if CO2 is present, a cloudy calcium carbonate precipitate will form.
Calcium hydroxide is not toxic at low concentrations. However, at higher concentrations, eye/skin contact, inhalation or swallowing of the aqueous calcium hydroxide solution can cause blindness, severe skin irritation, chemical burns, or lung damage.
Reference: