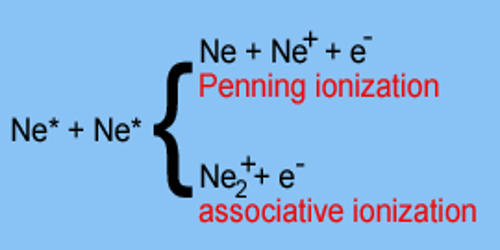
Carbonate Ester
Definition
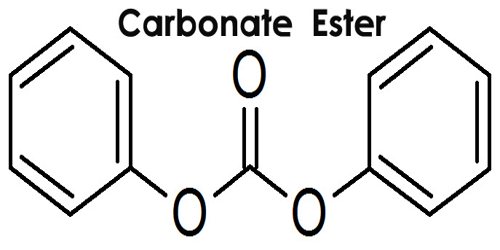
Carbonate ester is a salt or ester of carbonic acid, containing the group CO3. The reaction of carbonic acid with a metal result in a salt, and the reaction of carbonic acid with an organic compound result in an ester, such as diethyl carbonate. The general structure of these carbonates is R1O(C=O)OR2 and they are related to esters R1O(C=O)R and ethers R1OR2 and also to the inorganic carbonates.
A number of carbonate prodrugs have been reported for the opioid antagonist naltrexone, whose phenolic group was esterified to yield a small series of alkyl O-carbonates (alkyl=methyl, ethyl, n-propyl, isopropyl, n-butyl, and n-pentyl). All prodrugs hydrolyzed to the parent drug when passing through sheets of human stratum corneum, with the methyl carbonate providing the highest drug flux, apparent permeability coefficient, and stratum corneum vehicle partition coefficient. Small carbonate esters like dimethyl carbonate, and ethylene and propylene carbonate are used as solvents. Dimethyl carbonate is a mild methylating agent as well.
Oleochemical Carbonates
Carbonates are well known to chemists as they represent an important class of organic compounds and among them oleochemical carbonates have interesting characteristics which make them candidates for many industrial applications. The advantages of oleochemical carbonates are obvious: they open a door to interesting new products with new properties and they have advantages in hydrolytic stability regarding to the corresponding esters. Also their toxicological behavior is very good, hydrolysis of carbonates leads only to carbon dioxide and the alcohols.
The polar nature of the carbonate moiety enables it to adhere strongly to metal surfaces. Thus, they are used as lubricant components which have a protective property for metal corrosion. Some C8 to C18 carbonates have been exploited in personal-care products (sunscreen, cosmetics), dioctyl carbonate being also used as emollient or solvent in UV-filter solutions. Extraction of metal ions (gold, silver, platinum) is improved by the use of the chelating properties of oleochemical carbonates when mixed with the metal-containing aqueous phase. Future developments will ensure a growing interest in these molecules.
Several oleochemical carbonate products are now established. They can be used in technical applications and in cosmetic formulations. For example dioctylcarbonate is an emollient with superior skin sensory profile for many cosmetic applications.
There are two main industrial ways of preparing carbonate esters: the reaction of an alcohol (or phenol) with phosgene (phosgenation), and the reaction of an alcohol with carbon monoxide and an oxidizer (oxidative carbonylation). Other carbonate esters may subsequently be prepared by transesterification.
Reference: