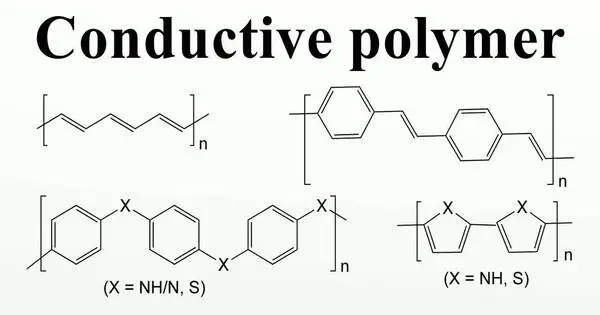
Organic polymers that conduct electricity are known as conductive polymers or intrinsically conducting polymers (ICPs). These are a type of organic material that has electrical conductivity while preserving some of the characteristics of typical polymers, such as flexibility and simplicity of processing. These compounds may be metallic conductors or semiconductors.
The fundamental advantage of conductive polymers is their ease of processing, primarily by dispersion. Conductive polymers are not thermoplastics, which means they cannot be thermoformed. Because of its potential applications in different industries, such as electronics, sensors, and energy storage, these materials have gotten a lot of attention in the domains of materials science, chemistry, and electronics.
Here are some key points about conductive polymers:
- Conductivity: Because they can transmit electricity, conductive polymers are unique among organic compounds. This conductivity is caused by the existence of delocalized π-electrons along the polymer backbone, which allows charge to flow.
- Conjugated Structure: The conjugated structure of these polymers, where alternating single and double bonds form a network of overlapping π-orbitals, is the essential property that gives them electrical conductivity. This opens up a channel for electron migration, allowing for electrical conduction.
- Doping: In their undoped, neutral form, conductive polymers are often insulating. Their conductivity, however, can be greatly boosted by a procedure known as “doping.” Doping is the introduction of dopant molecules or ions into the polymer structure, which results in the injection of charge carriers (either electrons or holes) and increases the conductivity of the polymer.
Applications:
- Organic Electronics: Conductive polymers have been used in organic electronic devices such as organic light-emitting diodes (OLEDs), organic solar cells, and organic field-effect transistors (OFETs).
- Sensors: They are employed in various sensor applications, including chemical and biological sensors, where changes in conductivity can be correlated with specific analytes.
- Batteries and Supercapacitors: Conductive polymers have been investigated for use in batteries and supercapacitors due to their ability to store and release charge efficiently.
- Antistatic Coatings: They are used in antistatic coatings for materials susceptible to electrostatic discharge, such as electronics components.
- Corrosion Protection: Conductive polymers can be applied as corrosion-resistant coatings in various industries.
- Actuators: Some conductive polymers can exhibit mechanical responses to electrical stimuli, making them suitable for use in artificial muscles and actuators.
Examples
Some commonly studied conductive polymers include polyaniline (PANI), polypyrrole (PPy), polythiophene (PT), and polyacetylene (PA). Each of these polymers has unique electrical and chemical properties that make them suitable for different applications.
Challenges
Despite their numerous advantages, conductive polymers have drawbacks such as instability in air and moisture, limited conductivity in comparison to metals, and problems in achieving exact control over their properties. Researchers are working hard to address these issues.
In conclusion, conductive polymers are an intriguing family of materials with numerous potential uses, particularly in the creation of flexible and lightweight electronic devices, sensors, and energy storage technologies. Ongoing research furthers our understanding of these materials and improves their performance in practical applications.