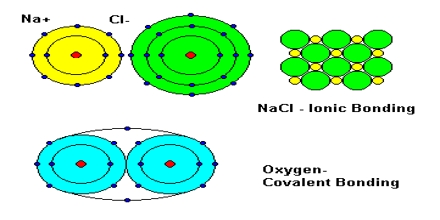
Basic purpose of this lecture is to present Ionic, Covalent and Metallic Bonding. Here briefly focus on Bond Formation: The positive sodium ion and the negative chloride ion are strongly attracted to each other. This attraction, which holds the ions close together, is a type of chemical bond called an ionic bond. Finally present Convalent Bonds: Some atoms are unlikely to lose or gain electrons because the number of electrons in their outer levels makes this difficult.