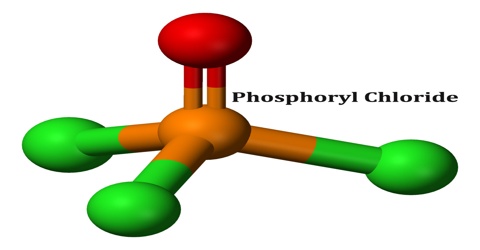
Phosphoryl Chloride
Definition
Phosphoryl chloride is an etherification and esterification agent for modification of food starch. It is a colourless liquid with the formula POCl3. It hydrolyses in moist air to phosphoric acid to release choking fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.
Phosphoryl chloride commonly called phosphorus oxychloride, which is a colorless fuming liquid with a pungent odor. Density 14.0 lb / gal. Very toxic by inhalation and corrosive to metals and tissue. Used in gasoline additives and hydraulic fluids.
Phosphoryl chloride can be prepared by various methods, notable example include:
The reaction of phosphorus pentachloride (PCl5) with phosphorus pentoxide (P4O10). The reaction can be simplified by chlorinating a mixture of PCl3 and P4O10, generating the PCl5 in situ. As the PCl3 is consumed, the POCl3 products become a solvent for the unreacted solid starting reagents:
6 PCl3 + 6 Cl2 → 6 PCl5
6 PCl5 + P4O10 → 10 POCl3
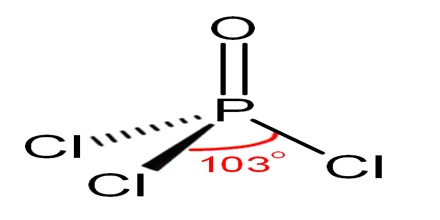
Structure and Properties of Phosphoryl Chloride
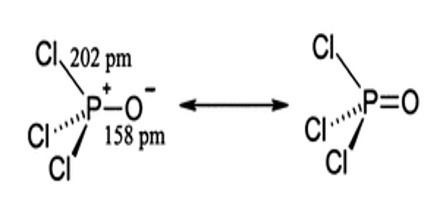
Phosphoryl chloride is tetrahedral in shape. It features three P-Cl bonds and one very strong P=O double bond, with an estimated bond dissociation energy of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is very dominant. The P=O bond does not resemble the π bond in a carbonyl group as in a ketone. The appropriate description of the P-O interaction is a matter of long discussion. Older textbooks favour a description that invokes participation of the d-orbitals on phosphorus. Some of these d-orbitals project toward the O atom, overlapping with p-orbitals on oxygen. More modern texts seem to favour a description where the P-O π bonding involves the sigma components of the P-Cl bonds.
POCl3 reacts with water and alcohols to give phosphoric acid or phosphate esters, respectively, for example
O=PCl3 + 3 H2O → O=P(OH)3 + 3 HCl
If the water is replaced by an alcohol, the trialkyl phosphate esters result. Such reactions are often performed in the presence of an HCl acceptor such as pyridine or an amine. If POCl3 is heated with an excess of a phenol (ArOH) in the presence of a Lewis acid catalyst such as magnesium chloride a triaryl phosphate ester is formed, for example:
3 C6H5OH + O=PCl3 → O=P(OC6H5)3 + 3 HCl
Phosphoryl chloride is a colorless liquid which fumes in air. With the freezing point of 1 °C and boiling point 106 °C, the liquid range is rather similar to water.
Uses of Phosphory Chloride
In the semiconductor industry, Phosphory Chloride (POCl3) is used as a safe liquid phosphorus source in diffusion processes. The phosphorus acts as a dopant used to create N-type layers on a silicon wafer.

In the laboratory, Phosphory Chloride is widely used as a dehydrating agent, for example the conversion of primary amides to nitriles. Similarly, certain aryl amides can be cyclised to dihydroisoquinoline derivatives using the Bischler-Napieralski reaction.
The most important use for phosphoryl chloride is in the manufacture of triarylphosphate esters (as described above) such as triphenyl phosphate and tricresyl phosphate. These esters have been used for many years as flame retardants and plasticisers for PVC. Meanwhile, trialkyl esters such as tributyl phosphate (made similarly from butan-1-ol) are used as liquid-liquid extraction solvents in nuclear reprocessing and elsewhere.
Reference: cs.mcgill.ca, pubchem.ncbi.nlm.nih.gov, wikipedia.