PNA: Peptide Nucleic Acid
Definition
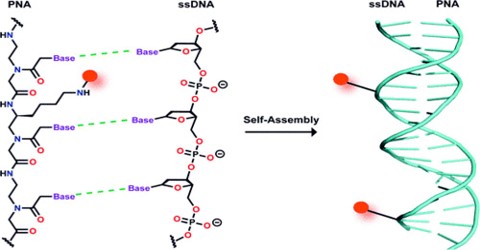
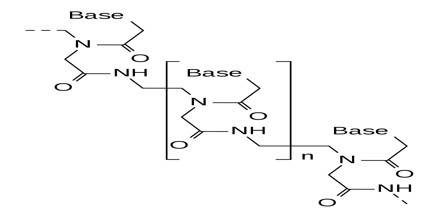
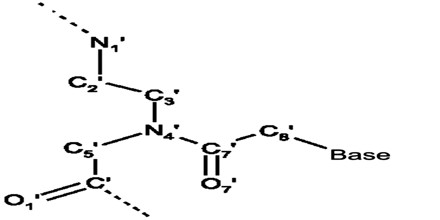
Peptide Nucleic Acid (or PNA) is a nucleobase oligomer in which the whole backbone is mainly replaced by N-(2- aminoethyl) glycine units. PNA is considered as DNA with a neutral peptide backbone due to negative charged sugar–phosphate backbone. It is stable chemically and biologically. Peptide bonds can make PNA readily conjugated with peptides, fluorescent dyes, and other useful molecules.
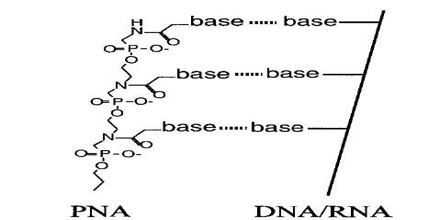
PNA is an extremely good structural mimic of DNA (or RNA), and PNA oligomers are able to form very stable duplex structures with Watson-Crick complementary DNA, RNA (or PNA) oligomers, and they can also bind to targets in duplex DNA by helix invasion. Therefore, these molecules are of interest in many areas of chemistry, biology, and medicine including drug discovery, genetic diagnostics, molecular recognition and the origin of life.
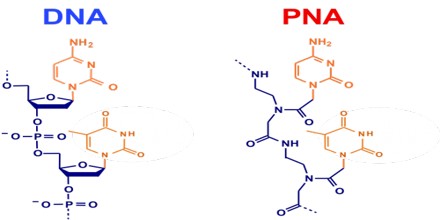
PNA was originally designed as a ligand for the recognition of double stranded DNA. The concept was to mimic an oligonucleotide binding to double stranded DNA via Hoogsteen base pairing in the major groove. Thus the nucleobases of DNA were retained, but the deoxyribose phosphodiester backbone of DNA was replaced by a pseudo-peptide backbone that according to computer model building was homomorphous with the DNA backbone. In theory a neutral (peptide) backbone should improve the triplex binding capability of the ligand, and we believed that the pseudo peptide backbone was a good chemical scaffold that would allow us to design recognition moieties that went beyond homopurine targets.
Synthetic peptide nucleic acid oligomers have been used in recent years in molecular biology procedures, diagnostic assays, and antisense therapies. Due to their higher binding strength it is not necessary to design long PNA oligomers for use in these roles, which usually require oligonucleotide probes of 20–25 bases.
Applications of Peptide Nucleic Acid (PNA)
A possible breakthrough may have come in efforts to develop PNA into gene therapeutic drugs. In eukaryotic systems, antisense activity of PNAs, as peptide conjugates has been reported in nerve cells and even in rats upon injection into the brain, and antisense activity has also been demonstrated in Escherichia coli. PNA hybridization technology has developed rapidly within in situ hybridization, and exciting new methods based on MALDI-TOF detection have also been presented.
Applications include alteration of gene expression – both as inhibitor and promoter in different cases, antigene and antisense therapeutic agent, anticancer agent, antiviral, antibacterial and antiparasitic agent, molecular tools and probes of biosensor, detection of DNA sequences, and nanotechnology.
Peptide Nucleic Acid or PNA has been hypothesized that the earliest life on Earth may have used PNA as a genetic material due to its extreme robustness, simpler formation, and possible spontaneous polymerization at 100 °C, while water at standard pressure boils at this temperature, water at high pressure—as in deep ocean—boils at higher temperatures. If this is so, life evolved to a DNA/RNA-based system only at a later stage. Evidence for this PNA world hypothesis is however far from conclusive.
Synthesis of Peptide Nucleic Acid (PNA)
Peptide Nucleic Acid or PNA oligomers had been synthesized by well established solid-phase peptide synthesis using Boc or Fmoc chemistry. Boc chemistry was originally used by PNA inventors and can be used with an automatic peptide synthesizer.
However, it has to use extremely hazardous trifluoroacetic acid repeatedly and cannot be used with an automatic DNA synthesizer. Fmoc chemistry uses milder reaction condition and can be used with a DNA synthesizer. However, it produces PNA oligomers with more impurities, some of which, such as generated by trans-acylation reaction, are extremely difficult to separate because the impurities are of the same molecular weight and other similar properties.
Recently Bts chemistry was developed by Panagene using self-activated building blocks. Bts chemistry produces purer crude PNA oligomers. More importantly the impurities are aborted oligomers because Bts chemistry does not add building blocks to a misbehaved product. Aborted oligomers are of smaller molecular weight and can be readily removed by HPLC purification.
Peptide Nucleic Acid (or PNA) could have been more widely used if the former licensed PNA supplier should not have had issues of long delivery time and inability to deliver certain PNA sequences. High quality PNA synthesized by Bts chemistry is commercially available in large and small volume with great sequence flexibility. Frequently requested PNA such as telomere probes are readily available and typical quantity of custom PNA is delivered in few weeks currently.
Peptide Nucleic Acids (or PNAs) are neutral and are commonly appended with positively charged lysine residues to enhance their solubility and binding affinity for nucleic acid targets. Thus obtained cationic PNAs very effectively interact with the designated duplex DNA targets in a sequence-specific manner forming strandinvasion complexes. Abibi et al. have reported that a typical range of salt concentrations used when working with strand-invading PNAs (10–20 mM NaCl) the PNA binding rates essentially do not depend on the presence of non-target DNA in the reaction mixture.