Potassium Nitrate
Definition
Potassium nitrate (KNO3) is a transparent, white, crystalline compound and strong oxidizing agent. It is used in gunpowder and fireworks, in making glass, and in fertilizer. It is also called saltpeter. It occurs as a mineral niter and is a natural solid source of nitrogen. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter or saltpetre.

Potassium nitrate is noncombustible and soluble in hot water but is not fully soluble in cold water. It is strong oxidizer and releases oxygen when it decomposes upon heating. Potassium nitrate is prepared commercially by the reaction of potassium chloride with sodium nitrate. In fires it produces toxic oxides of nitrogen.
Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of gunpowder (black powder) and has been used since the Middle Ages as a food preservative.
Production and Properties of Potassium Nitrate
Potassium nitrate fertilizer sometimes referred to as nitrate of potash or NOP is typically made by reacting potassium chloride (KCl) with a nitrate source. Depending on the objectives and available resources, the nitrate may come from sodium nitrate, nitric acid, or ammonium nitrate. The resulting KNO3 is identical regardless of the manufacturing process. Potassium nitrate is a chemical compound containing potassium and nitrate ions. It is an ionic salt.
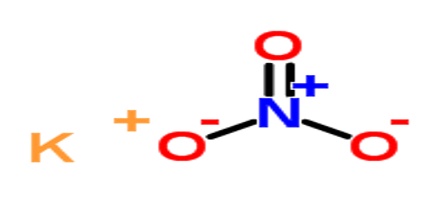
Industrially, it is prepared by a double displacement reaction between sodium nitrate and potassium chloride as follows:
NaNO3 (aq) + KCl (aq) –> NaCl (aq) + KNO3 (aq)
Potassium nitrate also used as an anodic inhibitor to prevent corrosion in reinforced concrete structures. It is very effective in preventing corrosion, but it is not used where there are existing aggregates with alkaline because they react with cement and cause extensive damage to the concrete.
Potassium nitrate can also be produced by neutralizing nitric acid with potassium hydroxide. This reaction is highly exothermic.
KOH (aq) + HNO3 → KNO3 (aq) + H2O (l)
Potassium nitrate is commonly sold as a water-soluble, crystalline material primarily intended for dissolving and applying with water or in a prilled form for soil application. Traditionally, this compound is known as saltpeter.
Applications of Potassium Nitrate
Potassium nitrate is used in fertilizers as a source of nitrogen and potassium – two of the macronutrients for plants. When used by itself, it has an NPK rating of 13-0-44. It is used as a water-soluble and virtually chloride-free source of nitrate-nitrogen and potassium nutrients.

Potassium nitrate is used in a wide variety of applications including glass manufacturing, explosives for mining and civil works, metal treatment, fireworks, and recently, as a means to drastically increase the efficiency of Concentrating Solar Power (CSP) plants. The use of solar energy helps to reduce the greenhouse effect produced by CO2 emissions from combustion engines into the atmosphere. Industrial nitrate use in this application has the potential to play a key role in future growth of demand.
Potassium nitrate is a well-known ingredient in the food industry, as a means to cure and preserve meats against microbial agents (e.g. Clostridium botulinum) and to maintain the desirable colour of meats and hard cheeses. It is also used to soften food and reduce cooking time when boiling beans and tough meat.
Potassium nitrate is a well-known product to desensitise sore teeth. It is therefore, a common ingredient in sophisticated toothpastes.
Reference: chemicalbook.com, corrosionpedia.com, kno3.org, dictionary.com, wikipedia.