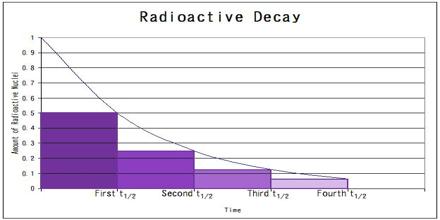
The rate of radioactive decay, that is the number of disintegrations per unit time, is proportional to the number of radioactive nuclei in the sample. The half-life is the time taken for the activity of a given amount of a radioactive substance to decay to half of its initial value. The mean lifetime (τ, “tau”) is the average lifetime of a radioactive particle before decay. The decay constant (λ, “lambda”) is the inverse of the mean lifetime.
Rate of Radioactive Decay