Sodium selenate is an inorganic compound with the formula Na2SeO4, not to be confused with sodium selenite. It is available as an anhydrous salt, a heptahydrate, and a decahydrate. These are white, water-soluble solids. It is sometimes used as a selenium fertiliser to supplement selenium in deficient soils and promote plant growth.
As a source of selenium, the decahydrate is a common ingredient in multivitamins and livestock feed. Some glass is made with anhydrous salt. Although selenates are far more toxic, many physical properties of sodium selenate and sodium sulphate are similar.
Properties
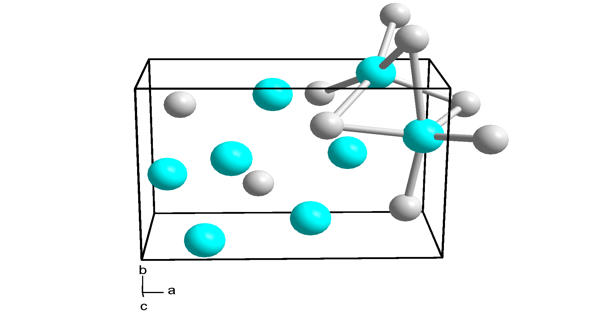
Sodium selenate typically exists as a white crystalline solid. It is highly soluble in water, meaning it dissolves readily in aqueous solutions. It is toxic and should be handled with care. It can cause severe health effects if ingested or if there is prolonged exposure.
- Chemical formula: Na2O4Se
- Molar mass: 188.947 g·mol−1
- Appearance: White or grey powder
- Density: 3.098 g/cm3
- Solubility in water: soluble
Production
Sodium selenate is produced by oxidation of selenium, first with nitric acid, producing selenous acid. The selenous acid is neutralized to form sodium selenite. The sodium selenite is oxidized in a basic medium hydrogen peroxide to form a selenate, which is then spray-dried.
Se + 2HNO3 → H2SeO3 + NO + NO2
H2SeO3 + Na2CO3 → Na2SeO3 + H2O + CO2
Na2SeO3 + H2O2 → Na2SeO4 + H2O.
It was prepared shortly after the discovery of selenium by Jöns Jacob Berzelius in 1817.
Application
Sodium selenate has numerous applications, the most common of which are in the manufacture of glass and ceramics. It is used as a fining agent in these materials, helping to remove bubbles and improve clarity. It is also used to make speciality glasses, such as those used in photovoltaic cells.
Environmental impact
Sodium selenate is a pollutant in the environment. It has the potential to contaminate water sources and soil, threatening aquatic life and plants. To reduce its impact, proper disposal methods should be used.