Acute Toxicity of an Unani Medicine
used in Dysentery
Dysentery broadly refers to infectious gastrointestinal disorders characterized by inflammation of the intestines, chiefly the colon. The World Health Organization (WHO) defines dysentery as any episode of diarrhea in which blood is present in loose, watery stools. Dysentery is spread among humans through contaminated food and water. Once a person is infected, the infecting organism lives in the intestines and is excreted through the stool of the infected person. With some infections, animals can also be infected and spread the disease to humans. Common bacterial causes of dysentery in the United States include infections with the bacteria Shigella and some types of Escherichia coli (E coli). Other less common bacterial causes of bloody diarrhea include Salmonella and Campylobacter infections. Dysentery is associated with environmental conditions where poor sanitation is prevalent. For example, childcare institutions in developing countries have higher rates of Shigella infection. Amebic dysentery, caused by the parasite Entamoeba histolytica, is most commonly found in tropical areas with crowded living conditions and poor sanitation.
The signs and symptoms of dysentery can last for five to seven days or even longer. The course of the illness varies among individuals, as do symptoms. Some people suffering with dysentery have mild symptoms, while others may have severe diarrhea with or without vomiting that can pose a risk of dehydration. Fortunately, dysentery can be treated with antibiotics and antiparasitic medications. Untreated dysentery can lead to severe dehydration. Severe dehydration and electrolyte imbalances can result in shock or coma and may be life-threatening.
Symptoms of Dysentery
Dysentery causes irritation and inflammation of the intestines that may result in a number of symptoms. The symptoms can vary in intensity among individuals. The most common symptoms of dysentery are related to disturbances of the digestive system and include:
- Abdominal bloating
- Abdominal pain
- Bloody diarrhea (may also be watery or with mucus)
- Cramping
- Flatulence
- Nausea with or without vomiting
As the dysentery infection progresses, other symptoms, including symptoms of dehydration, may develop. Other possible symptoms include:
- Decreased urine output
- Dry skin and mucous membranes (such as dry mouth)
- Feeling very thirsty
- Fever and chills
- Muscle cramps
- Muscle weakness (loss of strength)
- Weight loss
Causes of Dysentery
The bacteria Shigella and E coli and the amoeba Entamoeba histolytica are the most common causes of dysentery. These organisms are present in the stool (feces) of infected people and animals. The Entamoeba histolytica may uneventfully reside in the colon, but if it attacks the colon wall, it can cause dysentery. People with weakened immune systems are also more likely to develop amebic dysentery.
Most commonly, dysentery is caused by drinking water or eating food from sources contaminated with feces containing the pathogens. Swimming in contaminated water may also result in dysentery. For this reason, dysentery occurs most frequently in people traveling to developing countries and in children who touch infected human or animal feces without proper hand washing.
Common causes of dysentery include:
Several organisms are known to cause dysentery, most commonly:
- Campylobacter
- Certain types of E coli
- Entamoeba histolytica
- Salmonella
- Shigella
Treatments
Antibiotics:
Antibiotic medications that are effective in the treatment of dysentery caused by bacterial organisms include:
- Ceftriaxone
- Ciprofloxacin
- Trimethoprim-sulfamethoxazole
The most common treatment for amebic dysentery caused by Entamoeba histolytica is metronidazole , an antiparasitic medication.
Unani Medicine in Dysentery
Dysentery or bloody flux is a disorder of the digestive tract which is characterized by the inflammation of the large intestine and is accompanied by severe colic pain, formation of ulcers and passage of liquid or semi formed stools. Mucus and blood can also be present in the feces.
Dysentery is caused by protozoa and bacilli and can be acute or chronic in nature. Acute dysentery is characterized by pain in the abdomen, diarrhea and loose motions. At times only yellowish white mucus and blood from ulcers is present in the feces. The evacuations are preceded by pain and tenesmus. The patient feels a constant desire to evacuate, although there may be nothing to expel except for mucus and blood. In dysentery all the digestive processes are disturbed and the secretions are either stopped or changed. The saliva becomes acidic and the gastric juice becomes alkaline.
Many antibiotics are used in dysentery & diarrhea disease. Besides these medicinal plants play a significant role to control the dysentery & diarrhea.
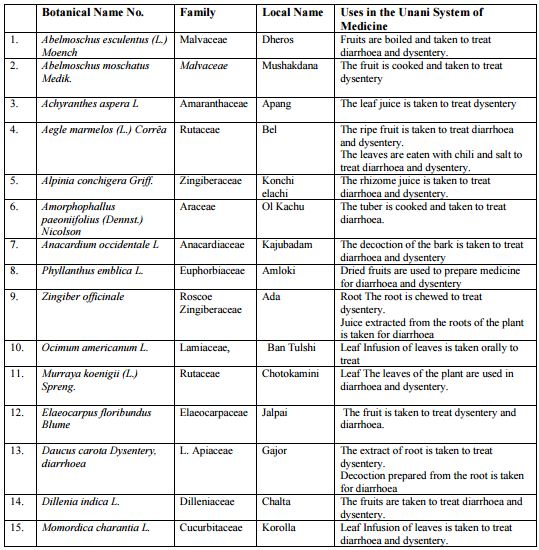
Table: Unani Medicine Plants used in dysentery in Bangladesh
Background of Unani Medicine in Bangladesh
In Bangladesh traditional system of medicine namely Unani & Ayurvedic medicine have been used in medical practice for thousands of years & have played a significant role in maintaining human health. Most of the people of Bangladesh meet their health needs with traditional medicine as they are unable to access modern allopathic medicine due to poverty.
Bangladesh National Formulary of Unani Medicine is compiled by the National Unani and Ayurvedic Formulary Committee and published by the Bangladesh Board of Unani and Ayurvedic Systems of Medicine, 38, Bangabandhu Avenue, Dhaka-1000 under the authority vested in the Board vide section13(J) of the Bangladesh Unani and Ayurbedic practitioners ordinance, 1983 in collaboration with the World Health Organization. Directorate of Drug Administration has issued Notification DA/Admin/1-10/96/6212 dated 19th October 1996 has issued license under Drug Act, 1940 and Rules there under and Drug(Control) Ordinance 1982 for local manufacturer and sale in Bangladesh.(Published Bangladesh Gazette#24 Part VI dated Thursday, June 11th 1998.) . Nowadays some western and developed countries are using traditional medicine for different health ailments to avoid the toxicity and side effects of synthetic and semi synthetic drugs. As traditional medicine is cheap, easily available and above all as because of natural origin, people can use this medicine with minimal health hazard and side effects. To strengthen the system of traditional medicine, the Government of Bangladesh has appointed thirty Unani and Ayurvedic Medical Officers in secondary levels hospitals under HPSP (Health and Population Sector program) from1999. They are providing health services with the existing health facilities without a uniform treatment guideline available to them so that the service could be rational and cost effective.
The government of Bangladesh has also taken step to appoint traditional Graduate physicians to the remaining district hospitals and all the Upazila Health Complexes to expand this system. 467 Herbal gardeners and 64 support personnel have already been recruited to assist the traditional medical officers and to take care the newly created 467 herbal gardeners in the district and Upazila Health Complex premises. They will also influence the people to use traditional medicine with the qualified herbal physicians and help to make the service easy and available for the rural and urban people. To maintain the quality, safety and efficacy of herbal medicine the preparation of pharmacopoeia for traditional medicine is under process under HNPSP program.
All the above activities have become possible due to the commitment of the Government and the support of World Health Organization.
Statement of Purpose
In the absence of an efficient primary health care system, traditional medicine occupies a central place in the provision of health care, especially among rural communities of developing countries. The strong historical bond between plants and human health is well substantiated by plant species’ diversity and related knowledge of their use as herbal medicines. In addition, it is attributable to the accessibility and affordability of herbal medicines.
Since time immemorial, man has made use of plants in the treatment of diseases. The pharmacopoeias of many countries of the world include even today a large number of drugs of plant origin. While it is true that purely synthetic compounds are being employed in increasing measure, in clinical practice, interest in the examinations of plants as potential source of new drug has never waned.
The task of revival of the old system of medicine however, is not an easy one at the present time. Advances in knowledge are so vast and occurs so fast in every time that an investigator is overwhelmed by the application of a new material with which he is confronted. There comes a time, however, when we feel like going back to the ancient page and leave where secrets remain to be revealed and unfold. We are fortunately endowed with a very rich flora, because of the size of our country and varieties of climatic and soil conditions, obtained in the different parts and as such there is wonderful opportunity for working on plant products
Another fortunate factor is that herbal medicines do not produce many side effects commonly seen after long-term administration of synthetic drugs, resulting in a revival of interest in their use all over the world in both developing and developed countries. In a recent survey it has been seen that 25-30% prescriptions, even in some developed countries contain plant ingredients.
With the fast growing demand for herbal drugs in the last two decades in every branch of medical care, it was considered expedient if not imperative to explore the therapeutic claims with least side effects of reported herbal drugs in reference monograph to serve the clinicians and scientists alike of both modern and herbal system of medicine.
Now a day’s most of the individuals are preferring to take herbal medicine to control their health, not only in prevention of diseases but also to treat them because natural remedies are somehow safer and more efficacious than remedies that are pharmaceutically derived. Hamdard Laboratories (Waqf) Bangladesh has been manufacturing and practicing Unani medicines since long time. These of unani medicines are widely prescribed for the treatment of various illnesses because many people cannot afford the use of expensive modern medicine.
One of such medicine is Marbelus syrup. It is an Unani Medicine of unique combination of Bael (Aegle marmelos) fruit and Connessi (Holarrhena antidysenterica) bark, which is highly effective in diarrhoea, dysentery (Amoebic & Bacillary dysentery), giardiasis and helminthiasis.
The bioactive compounds of Aegle marmelos are effective in diarrhoea, dysentery and peptic ulcer. Marbelus syrup relieves dysentery, bacillary dysentery, piles and diarrhoea. It relieves acute stomachache and duodenal inflammation. The bioactive compound of Holarrheana antidysenterica destroys amoeba. Now it has become imperative to conduct research on herbs to find out the toxicity and effectiveness of drugs for the benefit of man and animals and discard the ineffective, toxic and worthless drugs.
Therefore, the present study was conducted to determine the toxicity of the drug Marbelus Syrup being manufactured and marketed by Hamdard Laboratories (Waqf) Bangladesh and to validate the claim that Unani medicines are safe. With this perspective the present study has planned to evaluate the toxicity by using rats.
The research work has been designed based upon the effect of Marbelus Syrup on the level of
- Total Serum Protein & Albumin
- Lipid profile
- Liver Function
- Kidney function
- Serum Uric Acid
Definition of Toxicity
Toxicity is defined as “the potential of a substance to exert a harmful effect on humans or animals, and a description of the effect and the conditions or concentration under which the effect takes place”.
In order to support an application for a clinical trial or for the registration of a new drug, it is necessary to satisfy legislation that requires that certain data should be produced from a variety of toxicological investigations that show the safety profile of the compound to which humans may be exposed. Therefore, in the majority of cases of evaluation of the toxicity of most substances, rodents and non-human primates are first used in preclinical animal safety studies before further studies are done in humans. These animals are mainly used because of their biological similarity to humans that allows them to be regarded as the suitable metabolic models for humans in a broad range of investigations
In general, toxicity testing methods can be divided into two categories: The first category comprises tests that are designed to evaluate the overall effects of compounds on experimental animals. Individual tests in this category differ from each other basically in regard to the duration of the test and the extent to which the animals are evaluated for general toxicity. These tests are classified as acute, prolonged and chronic toxicity tests. The second category of tests consists of those that are designed to evaluate specific types of toxicity in detail. The prolonged and chronic tests do not detect all forms of toxicity, but they may reveal some of the specific toxicities and indicate the need for more detailed studies. Thus, this second category of tests has been developed for the determination of effects of compounds on the fetus in a pregnant animal (teratogenic tests), on the reproductive capacity of the animals (reproduction tests), on the genetic system (mutagenic tests), for the determination of the ability of agents to produce tumors (tumorigenicity and carcinogenicity tests), etc.
In this report, the focus was on the first category of toxicity testing methods, viz. acute toxicity tests. The primary concern was to determine how toxic the Unani Medicine might be after acute administration to rat.
Acute Toxicity
Acute toxicity has been defined as “the ability of a substance to cause severe biological harm or death soon after a single exposure or dose; or any poisonous effect resulting from a single shortterm exposure to a toxic substance”. An acute toxicity test is a single test that is conducted in a suitable animal species and may be done for essentially all chemicals that are of any biologic interest. Its purpose is to determine the symptomatology consequent to administration of the compound and to determine the order of lethality of the compound. The test consists of administering the compound to the animals on one occasion.
Furthermore, acute toxicity tests are those designed to determine the effects, which occur within a short period after dosing. They serve to establish the lethal dose range of the test substance and provide prompt warning if a highly toxic compound is being dealt with.
They also provide information on the limiting toxicity arising from the pharmacological effects of the compound on target organs and, often, on the maximum dose to be used in subsequent sub-acute studies (chronic studies). This latter information is particularly important for predicting the amount of chemical required for future toxicological studies.
The initial procedure, in an acute toxicity test programme, is to test a series of rangefinding single doses of the compound in a single animal species. This necessitates selection of a route of administration, preparation of the compound in a form suitable for administration via the selected route and selection of an appropriate experimental animal species. Generally, even if the intended use of the compound does not involve the oral or parenteral routes, at least the oral route is used, in addition to other routes, so that comparison with other related compounds can be made. Additionally, such testing may also indicate the viability of the oral route for use in subsequent, more extensive and prolonged toxicity studies. Normally, the significance and use of the data that are obtained are limited to those routes of administration that were used in the actual experiment.
As usual, all initial acute toxicity tests are performed on either rats or mice because of the low cost, the availability of the animals, and the fact that abundant reference toxicological data for many compounds in these species are available. In addition, these animals generally metabolize compounds in a similar manner to humans and the compounds (including metabolites) may have similar pharmacodynamics in the animals and humans. Before the experiment is performed, a total number of animals of similar body weight and same sex, or equal numbers of both sexes, are selected and randomly assigned to test (treatment) and control groups. After exposure to single doses of the test compound (or treatment), the animals are monitored for a minimum of 24hrs for any clearly recognized effect (such as changes in locomotor activity; bizarre reactions; sensitivity to pain, sound and touch; changes in social interaction; aggressive behavior; convulsions; paralysis, etc.) seen, as an index of toxicity, shortly or/and consistently after the administration of the chemical. However, the most easily recognized and certainly the most significant of effects is that of death and this outcome is usually used as a primary measure of acute toxicity. If the animals appear to be healthy at the end of 24hrs, they are monitored at daily intervals for at least a further one to two weeks for the appearance of delayed toxicity.
In the rodents, three types of acute toxicity studies may be performed. For the first type of study, it is usual to establish the maximum tolerated dose (i.e. the highest dose after which the animals recover completely from all effects of the chemical) and the minimum lethal dose for the compound (or treatment). The second type of study is the single dose study to establish the target organ(s) for toxicity while the third type of study is for the determination of the precise LD50 or median lethal dose. The results of the latter type of study may be required, in most countries, for a clinical trial’s certificate or even for a product licence. Usually, to establish a LD50, at least four dose levels are used, with 5-10 male animals plus 5-10 females per treatment group.
The animals are given a single dose of test compound and, at the end of 14-days observation period, the major organs and abnormal tissues of the surviving animals are collected and subjected to histopathological investigation.
The LD50 and its confidence limits are calculated from the lethality data using probit analysis.
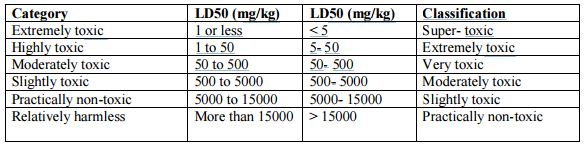
Since a great range of concentrations or doses of various chemicals may be involved in the production of harmful effects, the LD50 has been used by some authors to devise categories of toxicity on the basis of the amounts of the chemicals necessary to produce harm. An example of such a categorization, along with the respective lethal doses, is given in the following Table.
Table: Classification of toxicity based on LD50 dose ranges.
Chronic Toxicity
Chronic toxicity is defined as “the capacity of a substance to cause poisonous health effects in humans, animals, fish and other organisms after multiple exposures occurring over an extended period of time or over a significant fraction of an animal’s or human’s lifetime ”. The purpose of the chronic toxicity test is to investigate the harmful effects that foreign compounds that are introduced to animals in repeated doses or in continuous exposure over an extended period of time may produce. The dose levels of compounds used usually range from a very low fraction of the therapeutically effective dose (i.e. somewhere in the range of the ED 50 for the compound in that species, or of the same order as the anticipated human therapeutic dose range) to doses that approach the maximum non-lethal dose (as established in rodent acute toxicity studies).
Different approaches to dose ranging studies are applied depending on the species being used. The procedures used can vary, but usually involve exposing the experimental animals (in typical group sizes of two to five animals/sex/group) to various doses of the test compound, i.e. from the maximal non-lethal dose (determined in the acute studies) down to doses in the pharmacological dose range. Clinical chemistry and haematological parameters are then measured at the start of the study (i.e. within 48 hours after the first dose) and at the end of the study, along with full histopathology analysis of all abnormal tissues plus the major organs (such as the heart, liver, kidneys, lungs and brain of the animals), at least at the end of the study.
Toxic effects
Toxic effects are defined as “harmful responses of a biological system to a toxic compound, and death of cells or the whole organism are the major response.”
In all the cases, the toxic effects are usually manifested either in an acute or a chronic manner, and occur mostly as a result of an acute or chronic exposure to toxic compound by oral ingestion, inhalation or absorption following skin contact. The toxic effects are seen as (1) signs or reflection of a disturbance of the normal activities of enzymes that perform essential biochemical roles in all forms of life; (2) alteration of the normal activities of plasma membrane that regulate the exchange of nutrients and metabolites between the cell and its surroundings and (3) the disturbances of other normal cell activities, e.g. RNA and DNA synthesis, growth, division and general metabolism at all levels of organization from sub-cellular to organ and organ system.
The way in which the toxic agent is introduced into the body also plays a significant role. Routes of administration
This term refers to the way in which drugs or compounds are introduced to animals or humans.
To evaluate toxicity of a compound in animals various routes may be used, but two most commonly used modes of administration for animals studies are via intraperitoneal injection or the oral route.
Intra-peritoneal injection
This is one of the methods of dosing, which may occasionally provide information about local as well as systemic toxicity. To give drugs by intraperitoneal dosing, the animal is laid on its back and the abdomen shaved. This area is thoroughly cleansed and, using an appropriate syringe and needle, the abdominal wall is punctured. To ensure minimal danger of perforation of abdominal viscera, the injection should be made rostral and lateral to the bladder at an angle of about 15º to the abdomen. The depth of penetration should not exceed 5mm.
Oral administration
The oral route is probably one of the most common means by which a chemical enters the body. In short, the oral administration is the form of administration involving the gastrointestinal tract, which may be viewed as a tube going through the body, starting at the mouth and ending at the anus. Although it is within the body, its contents are essentially exterior to the body fluids. Most orally administered chemicals can otherwise have a systemic effect on the organism only after absorption has occurred from the mouth or the gastrointestinal tract. Oral administration of chemicals that are rapidly absorbed from the gastrointestinal tract would theoretically expose the liver to concentrations of the agent that would not be obtained if other routes of administration were used. Furthermore, if a compound entered the enterohepatic cycle, at least a portion of the compound would be localized in the organs involved in the cycle. Compounds that are known to be toxic to the liver would be expected to be more toxic following oral administration on repeated occasions, whereas their administration by other routes may be less hazardous.
Current use and importance of herbal medicines
A World Health Organisation survey indicated that about 70-80% of the world’s populations rely on non-conventional medicine mainly of herbal sources in their primary healthcare. This is especially the case in developing countries where the cost of consulting a western style doctor and the price of medication are beyond the means of most people Bangladesh possesses a rich flora of medicinal plants. Out of the estimated 5000 species of different plants growing in this country more than a thousand are regarded as having medicinal properties. Use of these plants for therapeutic purposes has been in practice in this country since time immemorial.
Continuous use of these plants as items of traditional medicine in the treatment and management of various health problems generation after generation has made traditional medicine an integral part of the culture of the people of this country. As a result, even at this age of highly advanced allopathic medicine, a large majority (75-80%) of the population of this country still prefer using traditional medicine in the treatment of most of their diseases even though modern medical facilities may be available in the neighborhood. Use of herbal medicines is thus, widespread in the world and in Bangladesh, but still very little is known about these medicinal plants.
A medicinal plant is factually any plant which in one or more of its parts contains substances that can be used for therapeutic purposes or which are precursors for the synthesis of direct therapeutic agents. On the other hand, the knowledge and practice of herbal medicine, is assumed to be an acquisition of hundreds years of trial and observation handed down from generation to generation verbally or in writing. Specifically, in African traditional medicine, a treatment usually consists of a complex mixture of several extracts or the active principles of plants and often only one of the ingredients is responsible for the claimed therapeutic activity.
Plants were once a primary source of all the medicines in the world and they still continue to provide mankind with remedies. Examples include “Quinine” obtained from Cinchona spp and used against malaria (antiprotozoal); “Taxol” from Taxus brevifolius,used as anticancer and “Atropine” from Datura spp, used as mydriatic and antispasmodic. Today, still about one– quarter of all marketed orthodox pharmaceutical medicines is either derived from plant sources or from derivatives of secondary plant metabolites. However, presently the rate for the successful discovery of new drugs from natural sources is not very promising. Several factors are responsible for this state of affairs of which the major factor is the triumph of syntheticchemistry by which natural extracts have, in the pharmaceutical industry, been replaced with synthetic molecules that often has no connection to natural products. Nevertheless, various chemicals and biotechnological products of plant origin are being screened by major multinational pharmaceutical industries in the hope of finding new cures for diseases that are better than current therapies in terms of effectiveness, selectivity and reduced side effects.
Other factors that sustain the low rate of success of herbal medicines as a potential source of new drugs are, firstly, the challenge and difficulty of acquiring well-documented plant material and the non-scientific process by which folk therapy systems operate, i.e. herbal medicine is often accused of being more based on faith. Secondly, and perhaps one of the greatest arguments against herbal medicine today, is the lack of scientific proof of its efficacy. Thirdly, witchcraft and the evil aspects associated with herbal medicine also discredit this form of medicine. Finally, further shortcomings of herbal medicine are the imprecise diagnosis that is often made by practitioners of this type of medicine and the absence of precise dosages for many herbal treatments. In recent years, however, it has been noted that plants are arguably poised for a comeback as sources of human health products. The hope for this comeback is rooted in the unique and newly appreciated properties of phytochemicals vis-à-vis conventional new chemicalentities-based pharmaceuticals, and are based on (1) the enormous propensity of plants to synthesize mixtures of structurally diverse bioactive compounds with multiple and mutually potentiating therapeutic effects; (2) the low-cost and highly scalable protein and secondary metabolite biomanufacturing capacity of plants; (3) the perception that because of the history of human use and co-evolution of plants and humans, phytochemicals provide a safer and more holistic approach to disease treatment and prevention. Although the above properties of plants have been known for a long time, the ability to better exploit the uniqueness of plant therapeutics was only recently acquired as a result of the dramatic advances in metabolic engineering, biochemical genomics, chemical separation, molecular characterization and pharmaceutical screening. A challenge for phytochemical-based botanical therapeutics is to integrate the ability to identify and genetically manipulate complex biosynthetic pathways in plants with better characterization of genetic targets for the prevention and treatment of complex diseases.
Notwithstanding the above, to date, the medicinal plant preparations are, in most cases, still used in their traditional dosage forms with ingredients that are poorly defined.
Traditional dosage forms and mode of administration of herbal medicines
When considering the traditional dosage forms of herbal medicine, several additional factors are also noteworthy. Firstly, the most common methods for administering traditional medicines are orally, sublingually, rectally, topically, nasally, smoking, steaming and bathing. Secondly, the method of preparation of the traditional dosage form is most of the time also critical, since it deals with the amount of fresh or dry plant material to be used, the addition of appropriate volumes of solvents such as water or alcohol and additional activities such as boiling for a specified length of time or partial burning to achieve a desired color. The additional activities are usually intended to neutralize certain toxins or to increase the extraction of the active compound in the aqueous form of medicines. The method of administration and the method of preparation of the herbal dosage form can be expected to have a significant impact on the effectiveness of the various traditional dosage forms of herbal medicine used. The following are examples of preparations commonly employed for therapeutic purposes via various specific routes of administration: (1) enemas, which are aqueous or oily solutions or suspensions, intended for rectal injection.; (2) the decoction, the extract of any plant drug obtained by boiling, as well as the infusion which is an extract prepared by macerating the crude drug for a short period of time in cold or boiling water, both intended for administration by mouth; (3) snuffs, preparations of finely powdered, dried medicinal plants that can be drawn up into the nostrils through inhalation;(4) inhalants, powder for licking, under the skin implantations, bath mixtures, poultices, balms, internal cleansing solutions and lotions are intended either for bathing with, rubbing into incisions, anointing, inhaling as smoke, licking or applying to skin or nibbling on.
Among other dosage forms not normally traditionally used but also very convenient are herbal tablets and capsules. Despite being quite useful, tablets may, however, contain fixed formulations that may not readily be adaptable to the individual’s specific needs. Capsules appear to be a more convenient form to take powdered plant drugs because they mask the unpleasant tastes or textures of the powdered forms of plant drugs and this is a distinct advantage for delivery of plant ingredients into the gastrointestinal tract. Given the variety of traditional dosage forms, methods of preparation and modes of administration used with herbal medicine, as well as the general non-professionalism many of the traditional medicinal practitioners display with respect to these activities and the impact that these processes may have on the effective use of herbal products, much concern has recently emerged over aspects of the efficacy, safety and quality of treatment methods and products obtained from herbal or natural sources. Particularly, there is a concern that many herbal practitioners continue to use plants (and dosage forms and routes of administration) for the treatment of diseases without any knowledge of the toxicity profiles or safety of their plant materials.
Toxicity aspects of use of herbal preparations
Currently, there is an ongoing world-wide “green” revolution which is mainly premised on the belief that herbal remedies are safer and less damaging to the human body than synthetic drugs. Many writers claim that it is assumed that “all things natural are good” and, generally, the extensive traditional use of herbal products is not assumed to be based on a comprehensive well documented logic, but rather on empirical wisdom accumulated over many years, often arrived at through trial and error and transmitted orally from generation to generation. This traditional methodology has enabled those herbal medicines producing acute and obvious signs of toxicity to be well recognized and their use avoided. However, the premise that “traditional use of a plant for perhaps many hundreds of years establishes its safety does not necessarily hold true”. The more sdubtle and chronic forms of toxicity, such as carcinogenicity, mutagenicity, and hepatotoxicity, may well have been overlooked by previous generations and it is these types of toxicity that are of most concern when assessing the safety of herbal remedies. Safety should be the overriding criterion in the selection of medicinal plants for use in health care systems. As with all forms of self-treatment, the use of herbal medicinal products also presents a potential risk to human health. As far as medicinal plants go, there are a few main categories of safety concerns. Firstly, there is the extrinsic or non-plant associated category where the patient may be at risk of toxicity as a result of exposure to (1) accidental or deliberate contaminants present in the herbal product or to (2) substitutes of herbal material. Secondly, we have the intrinsic or plant associated category of safety concerns. In this category, the patient may be exposed to potentially toxic substances naturally present in the herbal products or materials. Thirdly, there is the category of safety concerns related to interactions when the use of synthetic prescription drugs is combined with herbal medicines.
Finally, another category of safety concerns arises where specific patient groups may be at risk, e.g. pregnant or nursing mothers, children and the elderly.
There can thus be several causes of toxicity with herbal products.
Causes of toxicity with herbal products
All chemicals may be considered toxic under certain conditions, e.g. even pure water when inhaled is rapidly absorbed across the lung alveoli to cause lysis of red blood cells. But some chemicals present a greater hazard than others. A large number of plants contain appreciable levels of biosynthetically produced chemical substances and many of these have either been reported to be toxic to humans or are predictably toxic based on extensive animal or in vitro studies.
Toxicity with medicinal plant products may arise in various ways, but in general two categories of causes can be distinguished: In the first category, as previously mentioned, the toxicity may be as a result of exposure to intrinsic ingredients of some medicinal plants. Examples of some more important classes of ingredients implicated here include: pyrrolizidine alkaloids, which are said to be hepatocarcinogens; aristolochic acid I, said to bemutagenic and carcinogenic; phorbol esters, which are tumor promoters and vesicant to the skin; carboxyactractyloside, a deadly toxic compound; amygdalin, a cyanogenic compound with many undesired effects; etc.
In addition, several studies conducted on flavonoids indicate that, besides their apparently beneficial health effects, they may also induce utagenicity and genotoxicity (e.g. quercetin) in both bacterial and mammalian experimental systems. The second category of causes of toxicity of herbal medicines is more extrinsic or non-associated with the plant active constituents. In this category, the toxicity is a result of exposure to plant products contaminated with excessive or banned pesticides, microbial contaminants, heavy metals or chemical toxins, or with substituted ingredients. The pesticide, heavy metal and microbial contaminants may be linked to the source, collection or processing of the herbal materials (e.g. in contaminated environments). Chemical toxins may arise due to incorrect storage conditions or chemical treatment during storage. Some of these environmental factors can be controlled by implementing standard operating procedures that lead to good agricultural, good laboratory, good supply and good manufacturing practices for producing medicinal products from herbal or natural sources.
Prevalence of toxicity with herbal products
Different retrospective studies done over the last 20 years indicated that the incidence of deaths occurring due to exposure to plants (as a proportion of total patients poisoned by traditional plant medicine) was about 1.5% in France, 5% in Belgium, 6.5% in Italy, 7.2% in Switzerland and 6% in Turkey. The total number of deaths due to exposure to plants throughout the world however, is very difficult to establish and must certainly be underestimated since all cases of such deaths were, from analytical and forensic points of view, not always well documented and thus, rarely published. Nevertheless, it seems that death due to plant poisoning might be more important than other causes of poisonings. For instance, in South Africa, 2% of the people admitted for acute poisoning died compared to 15% of the patients poisoned with traditional plant medicine. From published reports, it appears that side effects or toxic reactions, of any form but associated with herbal medicines, are rare. This may be because herbal medicines are generally safe, that adverse reactions following their use are underreported, or because the side effects are of such a nature that they are not reported.
There is, therefore, a need for the public to have an understanding of the risks posed by herbal medicines so as to ensure that such products are used safely. Particularly, as highlighted above, the safety of some herbal products is compromised by lack of suitable control of the quality of herbal medicines and the absence of appropriate herbal use information for patients. To appropriately inform and protect the public, the herbal products must, however, first be evaluated for its toxicity.
Evaluation of herbal toxicity
Herbal toxicity can be evaluated by (1) observing human or animal populations exposed to the plant material, (2) administering the plant medicine to animals under controlled 20 conditions and observing the effects (in vivo) and (3) exposing cells, sub-cellular fractions or single-celled organisms to the plant material (in vitro). In this report the focus is more on the in vivo model.
In such cases, the accidents resulting from this type of exposure may, if well monitored and recorded (i.e. by measuring substances and their metabolites in body fluids and using biological indices of pathological change), provide important information about the toxicity of a plant material in humans. However, acquiring such data is often difficult and rarely complete, and the latter is the main reason why procedures for animal toxicity testing have been maintained as a successful alternative for evaluating the harmfulness of compounds for humans. As previously reported in sections 2.1.2 and 2.1.3, to date, the majority of data on the toxicity of chemicals, including drugs and herbal medicines, is gained from experimental studies done in animals (in vivo). The data so acquired are used for the risk assessment and safety evaluation of drugs (or herbal medicines) prior to human exposure. Because animal tests can be carefully controlled with the exact known doses being used, the quality of the data obtained is generally reliable. The number of animals used should be enough to allow statistical significance to be demonstrated and the application of humane conditions and proper treatment of the animals are essential, for scientific as well as ethical reasons, to help ensure that the data are reliable and robust. In a toxicity study, the animal species selected will depend partly on the type of toxicity test, existing data available and also on ethical and financial considerations. The most common species used are rats and mice for reasons of size, accumulated knowledge on 21 these species and cost, besides the similarity of their metabolism to that of humans In the present study, the concern was on the evaluation of the toxicity of Marbelus Syrup after the administration of the syrup (contains bioactive compounds-Aegle marmelos & Holarrheana antidysenterica) to rats.
As part of the effort of quality assurance of herbal products, scientific experiments were to be carried out to assess the safety of this medicinal plant using the rat as an alternative mammalian species.
Discussion
Herbal medicine, a natural remedy has become universally popular in primary healthcare, particularly in developing counties. Herbal sources are presumed to be safe without any compromising health effect, thus widely used as self medication. The use of herbal preparations as a treatment of diseases is very common. In Bangladesh, rural communities used herbs as food and traditional medicine. Marbelus syrup containing Aegle marmelos & Holarrhena antidysenterica has been used traditionally by some population as sarbat, dysentery & diarrhea. Some of these usages have been studied in in vitro and in animal model. Total alcoholic, total aqueous, whole aqueous and methanolic extracts were collected from the leaves of A. marmelos and studied in experimental rats for their toxicity. No histopathological changes were found when extracts of A. marmelos were administered intraperitoneally for 14 days successively at the dose of 50 mg/kg body wt. The collected data demonstrate that the extracts of the leaves of A. marmelos have a high margin of drug safety. Since the Aegle marmelose extract has previously been shown to be pharmacologically active (hypoglycemic) when orally administered to rats at the minimum active dose of mg/kg body wt, one may conclude that the active compounds present in the Aegle marmelose extract exhibit a rather low acute oral toxicity profile. The extract of Holarrhena antidysenterica has no toxic effect in research by the monitoring of GOT (Serum aspartate glutamate transferase) and GPT (Serum alanine glutamate transferase) activities in liver and kidney. Scientific evidence for their efficacy is widely studied but systemic safety studies are lacking. Therefore it is essential to evaluate the toxicity of the Marbelus syrup animals to ensure of its safety. The Marbelus syrup did not affect the body weight of the treated rats when compared to the control rats. Food and water intake of the treated rat and control group were similar. The increase in body weight of both group weekly were about 11 g, which considered normal and gradually as observed in SD rats of similar age group. The single oral dose of the Marbelus syrup did not produce mortality or significant changes in the body weight, food and water consumption. In the female rats there was significant (p=0.045) increase (16.40%) in total protein where albumin content was increased (1.56%) but not significantly (p=0.458) different from their corresponding control values. The decrease in urea content (0.80% decrease; p=0.691) & uric acid content was decreased by 12.54%; p=0.203 which was not statistically significant. The increase in both HDL cholesterol content (10.13% increase; p=0.46) & total cholesterol content (14.89% increase; p=0.23) was not statistically significant. It was observed that about 13.44% increase in triglyceride content of treated female rats in comparison to their control female rats which was statistically significant(p=0.030). Liver profiles were not significantly different as compared to the control except for total protein value. Triglycerides level in the treatment rats was significant different as compared to the control rats. Renal profile such as urea, uric acid were all normal as control group and indicated that there were no renal damage caused by Marbelus syrup to the rats.
Conclusion
It was concluded that the acute toxicity study of Marbelus syrup at 2000 mg/kg BW administered orally to Sprague Dawley rats did not caused any death or acute adverse effect on the clinical observation and mortality to the treatment rats. However, from the blood investigation, it showed that Marbelus Syrup consumption may cause dehydration as demonstrated by increased in total protein level. Other parameters except triglyceride were normal. No-observed-adverse-effect-level (NOAEL) of the Marbelus syrup is considered to be up to 2000 mg kg-1 day-1 for 14 days in rats. Thus, the lethal oral dose of Marbelus syrup is classified under category five, which is not at or below 2000 mg kg-1. This study provides valuable data on the toxicity profile of Marbelus syrup of Aegle marmelos & Holarrhena antidysenterica that would be useful in further pharmacological studies.