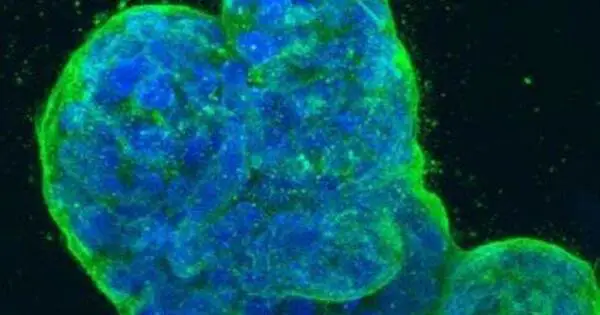
Cancer cells frequently experience a metabolic shift known as the Warburg effect. Cancer cells choose glycolysis over oxidative phosphorylation (aerobic respiration) for energy synthesis, even when oxygen is present. This metabolic change enables them to rapidly produce ATP (adenosine triphosphate) and other necessary building blocks for cell development. Increased glycolytic activity is characteristic of many cancer cells, including breast cancer.
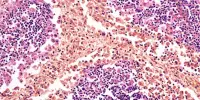
Breast cancer cells absorb and consume the matrix around them to overcome hunger, according to a new study published in the open-access journal PLOS Biology by Elena Rainero of the University of Sheffield, UK, and colleagues. The discovery elucidates a previously unknown process of cancer cell survival and may provide a new target for therapeutic development.
Cells in the breast, including tumor cells, are embedded in an extracellular matrix (ECM). Nutrients are rare in the ECM due to reduced blood flow, and this scarcity grows as tumor cells develop. Nonetheless, they continue to expand, prompting the authors to study how tumor cells obtain the raw elements required for growth.
Our results indicate that breast cancer cells take advantage of nutrients in the extracellular matrix in times of nutrient starvation and that this process depends on both macropinocytosis and metabolic conversion of key amino acids to energy-releasing substrates.
Elena Rainero
To do so, they seeded breast adenocarcinoma cells into either collagen (a major component of the ECM) or a commercial matrix preparation, or onto plastic, with or without certain critical amino acids. Without those amino acids, cells on plastic fared poorly compared to those in one or the other matrix. Similar results were seen with other matrix models – the tumor cells were able to overcome the reduction of amino acids when surrounded by matrix.
Next, by fluorescently labeling the collagen and watching its journey through the cell, the authors showed that the cells took up ECM and broke it down in digestive compartments called lysosomes; when the ECM was chemically treated to cross-link its components, the cells were unable to ingest it. Further investigation indicated that uptake was through an ingestion process called macropinocytosis, in which the cell engulfs large quantities of extracellular material.
What happened to the tumor cells afterward? Analysis of their metabolome revealed that the acquisition and breakdown of two amino acids, tyrosine and phenylalanine, dominated the metabolic alterations caused by fasting. The authors pointed out that these two can be used as raw materials for energy production via the mitochondrial tricarboxylic acid (Krebs) cycle. Cell growth was considerably reduced when HPDL, a key enzyme in the phenylalanine-to-TCA pathway, was knocked down. Blocking or lowering the expression of HPDL or the macropinocytosis promoter PAK1 lowered tumor cells’ ability to move and infiltrate adjacent tissue.
“Our results indicate that breast cancer cells take advantage of nutrients in the extracellular matrix in times of nutrient starvation, and that this process depends on both macropinocytosis and metabolic conversion of key amino acids to energy-releasing substrates,” Rainero stated in a press release. “HPDL-mediated metabolism of tyrosine and phenylalanine could represent a metabolic vulnerability of cancer cells thriving in a nutrient deprived microenvironment.”
The authors add, “This study identified a novel mechanism employed by breast cancer cells to survive in the challenging environment they are in within tumours. As sources of food are scarce, cancer cells gain the ability to eat and digest components of the matrix around them. Here we have identified a key metabolic process that the cells need to be able to take advantage of the matrix, which could represent a novel therapeutic target.”