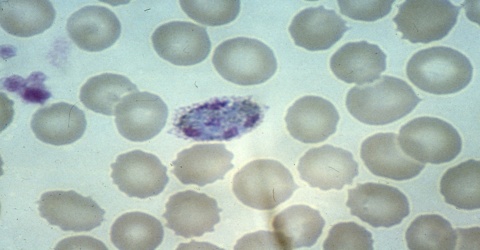
Malaria – Plasmodium ovale
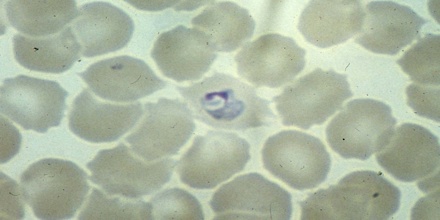
Plasmodium ovale was the last of the malaria parasites of humans to be described. The pronounced stippling of the infected erythrocyte and its tertian periodicity led early investigators to consider it a variant form of Plasmodium vivax. In 1900, Craig described a malaria parasite in the blood of American soldiers returning from the Philippines that had peculiar morphological characteristics and a tertian fever pattern. It is possible that he was describing infections with P. ovale. Macfie and Ingram in 1917 described a parasite in the blood of a child in the Gold Coast that may also have been P. ovale. Subsequently, Stephens observed in the blood of an East African patient some erythrocytes that were oval and with fimbriated edges. In 1922, he published a full description of the forms in the blood and named the parasite P. ovale in recognition of the oval shape of some of the infected erythrocytes.
Plasmodium ovale is primarily concentrated in sub-Saharan Africa and islands in the western Pacific. However P. ovale has also been reported in the Philippines, eastern Indonesia, and Papua New Guinea, as well as Bangladesh, Cambodia, India, Thailand and Vietnam.
In several studies, the reported prevalence of P. ovale was low relative to other malaria parasites, with fewer than 5% of malaria cases being associated with P. ovale infection. Higher prevalences of P. ovale are possible under certain conditions, as at least one study in Cameroon found the prevalence of P. ovale infection to be greater than 10%.
Life History about Plasmodium ovale
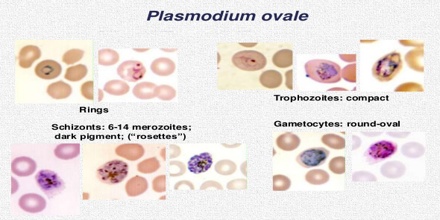
Plasmodium ovale has developmental cycles in the human host and in the vector mosquito. Eventually, many hundreds of merozoites are produced. Upon release, these merozoites invade reticulocytes and initiate the erythrocytic cycle. The development of some of the parasites in the liver cells is delayed or suspended as hypnozoites, occasionally for many months. Following a developmental cycle in the erythrocyte that lasts, on average, 49 h, from 8 to 20 merozoites are released to reinvade other erythrocytes. As with other species of Plasmodium that infect humans, some of the merozoites that invade erythrocytes develop into two forms of gametocytes.
During feeding, mosquitoes take up both microgametocytes and macrogametocytes. Within the gut of the mosquito, exflagellation of the microgametocyte occurs, resulting in the formation of up to eight microgametes. Following fertilization of the macrogamete, a mobile ookinete is formed that penetrates the peritropic membrane surrounding the blood meal and travels to the outer wall of the midgut of the mosquito.
There are situations where some of the sporozoites do not immediately start to grow and divide after entering the hepatocyte, but remain in a dormant, hypnozoite stage for weeks or months. The duration of latency is variable from one hypnozoite to another and the factors that will eventually trigger growth are not known; this explains how a single infection can be responsible for a series of waves of parasitaemia or “relapses”.
Symptoms of Plasmodium ovale
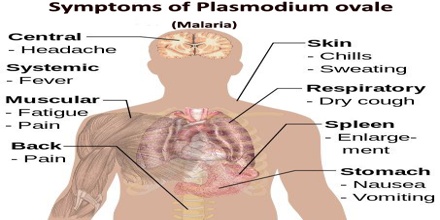
Humans are the only natural hosts for P. ovale. Much of what is known about this parasite was obtained during malaria therapy of naïve patients over 60 years ago. The prepatent period is the interval between sporozoite inoculation and the first detection of parasites in the peripheral blood.
In humans, symptoms generally appear 12 to 20 days after the parasite has entered the blood. In the blood, the parasite’s replication cycle lasts approximately 49 hours, causing tertian fever which spikes approximately every 49 hours as newly replicated parasites erupt out of red blood cells. Mean maximum parasite levels have been found to be 6,944/microl for sporozoite-induced infections and 7,310/microl for trophozoite-induced infections.
In some cases, relapse may occur up to 4 years after infection.
A retrospective examination of records from induced infections indicated that 47.1% of the fever episodes were ≥ 104°F. Patients reinfected with P. ovale rarely had fevers ≥ 104°F. An examination of fever episodes for 30 patients infected via sporozoites and 60 patients infected by the inoculation of parasitized erythrocytes indicated maximum fevers ranging from 102.0o to 107.0°F and 103.8o to 107.8°F, respectively. Mean maximum fevers were 103.3o and 105.4°F, respectively. For all patients, there were an average of 10.3 fever episodes of ≥101 and 4.5 fever episodes of ≥104°F.
Treatment of Plasmodium ovale
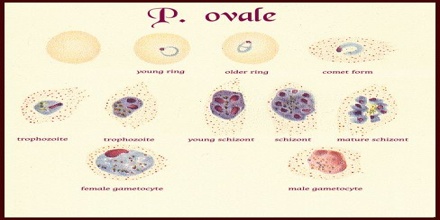
The microscopic appearance of P. ovale is very similar to that of P. vivax and if there are only a small number of parasites seen, it may be impossible to distinguish the two species on morphological grounds alone. There is no difference between the medical treatment of P. ovale and P. vivax, and therefore some laboratory diagnoses report “P. vivax/ovale”, which is perfectly acceptable as treatment for the two are very similar. Schüffner’s dots are seen on the surface of the parasitised red blood cell, but these are larger and darker than in P. vivax and are sometimes called James’ dots or James’ stippling. About twenty percent of the parasitised cells are oval in shape (hence the species name) and some of the oval cells also have fimbriated edges (the so-called “comet cell”). The mature schizonts of P. ovale never have more than twelve nuclei within them and this is the only reliable way of distinguishing between the two species.
Standard treatment is concurrent treatment with chloroquine and primaquine. The combination atovaquone proguanil may be used in those patients who are unable to take chloroquine for whatever reason.