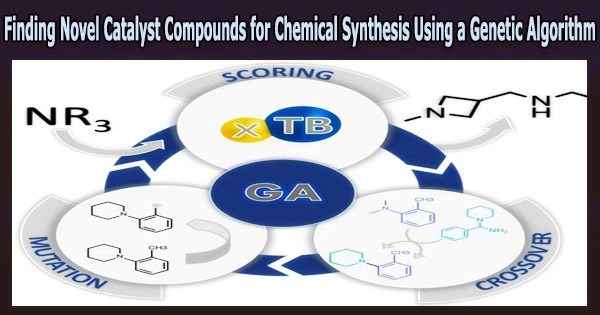
A genetic algorithm is a search and optimization technique inspired by the principles of natural selection and genetics. It is a type of evolutionary algorithm that is used to find optimal solutions to complex problems that are difficult to solve using traditional methods.
An organic catalyst with superior performance than existing catalysts has been found by a research team using a computational approach influenced by evolution. Angewandte Chemie International Edition, the group reports that a genetic algorithm recommended new, catalytically active molecular structures for a common reaction in chemical synthesis.
According to the experts, the technique might be used more generally in the quest for superior molecular catalysts. In several areas of chemistry, machine learning systems can already predict material properties and molecular structures with great accuracy.
Although creating novel catalysts for chemical reactions is presently one of the most significant objectives in chemical research, automating the search for new and improved catalysts hasn’t been achievable to yet. Faster and simpler reactions with lower energy requirements and fewer byproducts are made possible by more effective catalysts.
The reason behind the difficulties encountered by automated systems when seeking new catalysts lies in reaction transition states, as Jan Halborg Jensen, Professor of computational chemistry at the University of Copenhagen, Denmark, and corresponding author for the study, explains.
Azetidines had never been considered as catalysts for the MBH reaction, so the algorithm made a genuinely novel discovery.
Professor Jan Halborg Jensen
This is so because catalysts have an impact on the transition state, or the point in a process where a product will form or not. Modeling is challenging due to the moment’s transitory nature and the intricacy of the structures generated by numerous molecules interacting at once.
To overcome this, Jensen and the team turned to a selection method based on the principles of evolution. A genetic algorithm was used to evaluate a set of starting molecules for fitness for catalyzing the Morita–Baylis–Hillman (MBH) reaction.
“Then you take the fittest molecules and mate them, which means that you cut the two parents in random places and recombine fragments from each parent,” Jensen explains. “If you do this enough times, the final population can look very different from the initial population, much like a chihuahua differs from its wolf ancestors.”
Hence, a four-membered azetidine ring, which was absent from the initial population, was present in the final molecules produced by the computer. The team then synthesized one of the computer-evolved azetidine candidates and tested it in the reaction, finding that it performed considerably better than the traditional catalyst, DABCO (1,4-diazabicyclo[2.2.2]octane).
“Azetidines had never been considered as catalysts for the MBH reaction, so the algorithm made a genuinely novel discovery,” says Jensen, highlighting the importance of computer-assisted discoveries in chemical research.
The crucial transition state for the relevant reaction must be understood, according to Jensen, in order to employ this technique in the future. He thinks that if this were known, genetic algorithms might be able to aid in the discovery of novel and enhanced organocatalysts.