Autism overview
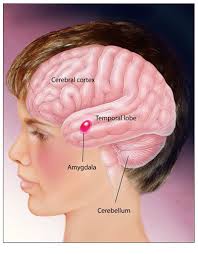
Autism is a potential threat to the present world. A great number of individuals have been suffering from autism worldwide. Autism is part of a spectrum disorders characterized by a triad of symptoms, including deficits in all aspects of social reciprocity; pragmatic communication deficits and language delays; and an assortment of behavioral problems, such as restricted interests, sensory sensitivities and repetitive behaviors [1]. Specific causes of autism have not yet been known and there’s no medication of it. There is no apparent core mechanism that could explain the assortment of symptoms found in autism; the triad of deficits suggests that a diverse set of neural systems are affected. Its early onset and familial pattern strongly suggest a biological basis, and, in fact, there are now substantial data implicating brain based as well as genetic mechanisms [2]. From the perspective of current heuristic models of the functional of typical children and adults, there is no apparent core mechanism that could explain the assortment of symptoms found in autism; the triad of deficits suggests that a diverse set of neural systems are affected. At the same time, however, the pattern of brain abnormality is discrete, because autism spares many perceptual and cognitive systems. This fact would seem to rule out any neurobiological explanation of autism that focuses on deficits of complex information processing as a necessary ingredient. A key feature of normal social functioning in human is the processing of faces, which allows people to identify individuals and enables them with the capacity to understand the mental state of others [3].
Autism is a neurodevelopmental disorder characterized by mild to severe qualitative impairment in communicative abilities and reciprocal interaction as well as repetitive and stereotyped behaviors. Autism is commonly considered a spectrum disorder (Autism spectrum disorder, ASD), ranging from profoundly isolated, mentally retarded individuals to intellectually brilliant individuals who only behaves oddly during social interaction. The question of whether autism is one or many diseases open, and some authors suggest that autism may be a syndrome (many separate diseases entities) rather than a spectrum (variation of a single disease) and that autism may be a final common phenotype expressed by many underlying diseases [23].
The prevalence of autism seems to have dramatically increased during the last decade, and recent studies [24] report that as many as 1 in 166 children may be affected by ASD. This is in strong contrast with literature from the 1970s and the 1980s that only reported up to 0.2% prevalence [23]. There are many possible reasons for this increase in prevalence, including change in diagnostic criteria and awareness.
One of the earliest symptoms of autism is the lack of attention to faces that can be apparent by one year of age, followed by deficits of joined attention [24]. Individuals of autism are impaired at using information from faces, such as gaze, facial expression and facial speech, to regulate social interaction. They have difficulties making social judgment relating to others and recognizing their emotions. Furthermore they fail to show their usual empathic reaction while other people demonstrate emotion of fear, pleasure or pain.
Ion Channel
Ion channels control the movement of ions across the neuronal membrane. These tiny anatomical structures make neurons excitable Ion channels passive, chemically-gated, or voltage gated are regionally located in the neuron. Ion channels allow the movement of charged particles, known as ions, across cell membranes. Ion channels function in a variety of biological pathways including the firing of neurons and muscle cells and the activation of immune cells. On channels are used to restore the balance of ions across a membrane. When open, ion channels allow charged molecules to move from an area of high concentration to low concentration without using energy. Ion channels serve as a counterbalance to active transport, a process whereby a cell uses energy to actively pump ions and other charged molecules across a membrane in order to establish ion gradients or alter the pH of an organelle to activate enzymes. There are two major types of ion channels:
- Voltage-gated ion channels
- Ligand-gated ion channels
Voltage-gated channel overview
In response to neuron stimulation, voltage-gated ion channels are opened, allowing the rapid and brief flow of sodium ions and potassium ions across the plasma membrane. Voltage-gated ion channels are similar to ordinary ion channels, but with one important difference; they are only open in response to a voltage change across the membrane. In a resting neuron, the voltage-gated ion channels are in the closed conformation and ions cannot pass through. In response to neuron stimulation, the voltage-gated ion channels open.
Voltage-gated calcium channels represent a heterogeneous family of calcium-selective channels that can be distinguished by their molecular, electrophysiological, and pharmacological characteristics. Voltage sensitive calcium channels mediate the rapid, voltage dependent entry of calcium into many types of nerve, muscle, and endocrine cells. In the nervous system, calcium entry contributes toward the electrical properties of neurons, modulates calcium-dependent enzymes and ion channels, controls calcium dependent gene transcription, and initiates the release of neurotransmitters and neuro-modulators. Electrophysiological analyses have categorized native calcium currents into two major classes that differ in their voltage activation properties. High voltage-activated (HVA) 1calcium channels represent a diverse family of channels that first activate at relatively depolarized potentials (usually. 240 mV) and that display distinct pharmacological characteristics (L-type, N-type, P/Q type, and R-type;) [1,2]. In contrast to activation for HVA calcium channels, low voltage-activated (LVA or T-type) calcium channels first activate with relatively small depolarization (between 280 and 260 mV) and have a poorly defined pharmacology. In addition to their distinct low voltage dependence of activation, T-type calcium channels also exhibit unique voltage-dependent kinetics, small single channel conductance, rapid inactivation, slow deactivation, and a relatively higher
Permeability to Ca21 compared with Ba21 [3]. The initial evidence for the existence of LVA calcium currents was obtained from neurons of the inferior olivary nucleus [4]. Subsequently, LVA currents were recorded from cells isolated directly from the dorsal root ganglia [5–9], pituitary [10, 11], and cardiac myocytes [12–16]. T-type channels are of interest, since they are responsible for rebound burst firing in central neurons and are implicated in normal brain functions such as slow wave sleep and in diseased states such as epilepsy [3, 17]. They are also thought to play a role in hormone secretion [18, 19] and smooth muscle excitability [20]. The biophysical characterization of T-type channels has been complicated by the presence of multiple types of calcium currents in neurons and other cells as well as a lack of specific pharmacological tools. In addition, there is considerable heterogeneity in the reported activation, inactivation, permeation, and pharmacology of neuronal T-type currents. T-type calcium channels represent the most recent calcium channel family to be described at the molecular level [21–25], although there remains little known concerning their biochemical composition.
1.3 Computational modeling overview
Modeling as a method of cognition has long-time/age long history. Man tried all sorts of means (verbal description, graphic display, use of mathematical symbols, physically and technically implemented models) to describe things in his environment and the phenomena observed by him. Models expressed, showed a significant features of real systems – objects.
A computational model is a computer program, run on a single computer, or a network of computers, that attempts to simulate an abstract model of a particular system. Computer simulations have become a useful part of mathematical modeling of many natural systems in physics (computational physics), astrophysics, chemistry and biology, human systems in economics, psychology, social science, and engineering. Simulation of a system is represented as the running of the system’s model. It can be used to explore and gain new insights into new technology, and to estimate the performance of systems too complex for analytical solutions.
The simulation system NEURON is a common research tool for constructing structurally and functionally realistic models of neuronal systems. NEURON allows the development of simulations at any level of complexity, from sub-cellular components to single cells, cellular networks, and system-level models. Focusing on an in vitrocell model of a single, acutely isolated thalamic neuron, we used the simulation environment to address and to analyze autism.
Computational models are created to simulate processes observed in a given system in order to understand their function. A realistic model allows predictions about the outcome of natural processes based on a specific set of input parameters. Therefore complicated systems, such as neurons of the central nervous system, are attractive and fascinating targets for modeling approaches, especially when some parameters are not experimentally accessible. Since scientists have begun applying modeling strategies, computational modeling software has grown at an accelerating rate. Supported by increasingly powerful hardware and software, growing numbers and varieties of computational models are being developed to support research, development, and education
Neuron-Basic Nerve Cell of Brain
Introduction
The human brain has the same general structure as the brains of other mammals, but is larger than any other in relation to body size. The human cerebral cortex is a thick layer of neural tissue that covers most of the brain. This layer is folded in a way that increases the amount of surface that can fit into the volume available. The pattern of folds is similar across individuals, although there are many small variations. The cortex is divided into four “lobes”, called the frontal lobe, parietal lobe, temporal lobe, and occipital lobe. Functions, such as vision, hearing, and speech, are distributed in selected regions .Some regions are associated with more than one function. major internal structures (bot-tom image). The forebrain is credited with the highest intellectual functions — thinking, planning and problem-solving. The hippocampus is involved in memory. The thalamus serves as a relay station for almost all the information coming into the brain. Neurons in the hypothalamus serve as relay stations for internal regulatory systems by monitoring information coming in from the autonomic nervous system and commanding the body through those nerves and the pituitary gland. On the upper surface of the midbrain are two pairs of small hills, colliculi, collections of cells that relay specific sensory information from sense organs to the brain. The hindbrain consists of the pons and medulla oblongata, which help control respiration and heart rhythms, and the cerebellum, which helps control movement as well as cognitive processes that require precise timing.
The Neuron
The neuron specialized cell designed to transmit information to other nerve cells, muscle, or gland cells, the neuron is the basic working unit of the brain. The brain is what it is because of the structural and functional properties of interconnected neurons. The brain contains between 1 billion and 100 billion neurons, depending on the species. Neuron transmits information through electrical and chemical signals. A chemical signal occurs via a synapse, a specialized connection with other cells. Neurons connect to each other to form neural networks. Neurons are the core components of the nervous system.
The neuron consists of a cell body, dendrites, and an axon. The cell body contains the nucleus and cytoplasm. The electrically excitable axon extends from the cell body and often gives rise to many smaller branches before ending at nerve terminals. Dendrites extend from the neuron cell body and receive messages from other neurons. Synapses are the contact points where one neuron communicates with another. The dendrites and cell body are covered with synapses formed by the ends of axons from other neurons.
Neurons signal by transmitting electrical impulses along their axons, which can range in length from a tiny fraction of an inch to three feet or more. Many axons are covered with a layered myelin sheath, which speeds the transmission of electrical signals along the axon. This sheath is made of specialized cells called oligodendrocytes in the brain and Schwann cells in the peripheral nervous system.
Nerve impulses involve the opening and closing of ion channels, which are selectively permeable, water-filled molecular tunnels that pass through the cell membrane and allow ions — electrically charged atoms — or small molecules to enter or leave the cell. The flow of these ions creates an electrical current that produces tiny voltage changes across the neuron’s cell membrane.
The Structure of the Neuron
A neuron has a cell body with a cluster of fibers called dendrites at one end. Those fibers, which look like the twisted branches of a tree, receive messages from other neurons. On the opposite end of the cell body is a long, slim, tube-like extension called an axon. The axon carries messages received by the dendrites to other neurons. The axon is considerably longer than the rest of the neuron. Although most axons are several millimeters in length, some are as long as three feet. Axons end in small bulges called terminal buttons, which send messages to other neurons. The messages that travel through a neuron are electrical in nature. Although there are exceptions, those electrical messages, or impulses, generally move across neurons in one direction only. Impulses follow a route that begins with the dendrites, continues into the cell body, and leads ultimately along the tube-like extension, the axon, to adjacent neurons. Dendrites, then, detect messages from other neurons; axons carry signals away from the cell body. Most axons are insulated by a myelin sheath,
Fig: Structure of the neuron
A protective coating of fat and protein wraps around the axon. The myelin sheath also serves to increase the velocity with which electrical impulses travel through axons. Those axons that carry the most important and most urgently required information have the greatest concentrations of myelin.
Where Neurons Meet: Bridging the Gap
There a chemical connection bridges the gap, known as a synapse, between two neurons A synapse is the junction between an axon and a dendrite. The gap between the axon and the dendrite is bridged by chemicals called neurotransmitters [20]. Just as the pieces of a jigsaw puzzle can fit in only one specific location in a puzzle, each kind of neurotransmitter has a distinctive configuration that allows it to fit into a specific type of receptor cell. When a nerve impulse comes to the end of the axon and reaches a terminal button, the terminal button releases a chemical courier called a neurotransmitter. Neurotransmitters are chemicals that carry messages across the synapse to a dendrite (and sometimes the cell body) of a receiving neuron. These chemical messengers move toward the shorelines of other neurons. Although messages travel in electrical form within a neuron, they move between neurons through a chemical transmission system.
ion channel-transport through cell membrane
Introduction
All the cells in the body must be supplied with essentials substances like nutrients, water, electrolytes, etc. And must get ride off many unwanted substances like waste materials, carbon dioxide, etc.
The structure of the cell membrane is well suited for the transport of substances in and out of the cell. The lipids and proteins of the cell play important role in the transport of various substances between ECF and ICF. The cell membrane is a lipid bi-layer in which large protein molecules, called integral proteins, are embedded. Some of the integral proteins contain watery pores called ion channels, through which charged particles, or ions, can pass. Ion channels control the movement of ions through the neuronal cell membrane.
Basic mechanism of transport
Two types of basic mechanism are involved in the transport of substances across the cell membrane.
- Passive transport mechanism
- Active transport mechanism
Passive transport
Passive transport is the transport of the substances along the concentration gradient or electrical gradient or both (electrochemical gradient). It is also known as diffusion or downhill movement. It does not need energy. Here the substances move from the region of higher concentration to the region of lower concentration. Diffusion is of two types namely, simple diffusion and facilitated diffusion. Simple diffusion of substances occurs either through lipid layer or protein layer of the cell membrane. The facilitated diffusion occurs with the help of the carrier proteins in the cell membrane. Thus the diffusion are classified into three categories
- Simple diffusion through lipid layer
- Simple diffusion through protein layer
- Facilitated or carrier mediated diffusion
Simple diffusion through lipid layer
The lipid layer of the cell membrane is permeable only to lipid soluble substances like oxygen, carbon dioxide, and alcohol. The diffusion through the lipid layer is directly proportional to the solubility of the substances in the lipids.
Simple diffusion through protein layer
The protein layer in the cell membrane is permeable to water soluble substances. Mainly electrolytes diffuse through the protein layer.
Protein channel or ion channel
Throughout the central lipid layer of the cell membrane there are some pores. The integral protein molecules of protein layer invaginate into these pores from either surface of the cell membrane. These pores are hypothetical pores and from the channel for the diffusion of water, electrolytes, and other substances, which cannot pass through the lipid layer.
Types of protein layers or ion channels
The characteristics feature of the protein channel is the selective permeability. That is each channel can permit only type ion to pass through it. Accordingly the channels are named after the ion which diffuse through these channel such as calcium channel, potassium channel etc.
Regulation of the channel
Some of the protein channels are continuously open and most of the channels are always closed. Continuously open channels are called ungated channel. The closed channels are called gated. These channels are opened only when required.
Gated channel
The gated channels are divided into three categories
- Voltage gated channel
- Ligand gated channel
- Mechanically gated channel
Ion Channels Are Functionally Unique
• Ion channels have specific functions, which are suggested by their locations on the neuron:
• Passive Channels.
Passive channels are responsible for the resting membrane potential.
• Chemically-gated Channels.
Chemically-gated channels are responsible for synaptic potentials, the incoming signals to the neuron.
• Voltage-gated Channels.
Voltage-gated channels are responsible for generation and propagation of the action potential, the outgoing signal from the neuron.
Figure: The gating of ion channels. There are three different kinds of ion channels, each opened by a different kind of stimuli. (From Alberts et al., Essential Cell Biology, Garland Publ, 1998, Fig 12-22, p. 390.)
Depolarization
Certain external stimuli reduce the charge across the plasma membrane.
- Mechanical stimuli (e.g., stretching, sound waves) activate mechanically-gated sodium channels
- Certain neurotransmitters (e.g., acetylcholine) open ligand-gated sodium channels.
In each case, the facilitated diffusion of sodium into the cell reduces the resting potential at that spot on the cell creating an excitatory postsynaptic potential or EPSP.
If the potential is reduced to the threshold voltage (about −50 mv in mammalian neurons), an action potential is generated in the cell.
Action Potentials
If depolarization at a spot on the cell reaches the threshold voltage, the reduced voltage now opens up hundreds of voltage-gated sodium channels in that portion of the plasma membrane. During the millisecond that the channels remain open, some 7000 Na+ rush into the cell. The sudden complete depolarization of the membrane opens up more of the voltage-gated sodium channels in adjacent portions of the membrane. In this way, a wave of depolarization sweeps along the cell. This is the action potential. (In neurons, the action potential is also called the nerve impulse.)
Hyperpolarization
Despite their name, some neurotransmitters inhibit the transmission of nerve impulses. They do this by opening
- chloride channels and/or
- Potassium channels in the plasma membrane.
In each case, opening of the channels increases the membrane potential by
- letting negatively-charged chloride ions (Cl−) IN and
- positively-charged potassium ions (K+) OUT
This hyperpolarization is called an inhibitory postsynaptic potential (IPSP) because it counteracts any excitatory signals that may arrive at that neuron. Although the threshold voltage of the cell is unchanged, it now requires a stronger excitatory stimulus to reach threshold. Example: Gamma amino butyric acid (GABA). This neurotransmitter is found in the brain and inhibits nerve transmission by both mechanisms:
- Binding to GABAA receptors opens chloride channels in the neuron.
- Binding to GABAB receptors opens potassium channels.
Classification by gating
There are over 300 types of ion channels in a living cell. Ion channels may be classified by the nature of their gating, the species of ions passing through those gates, the number of gates (pores) and localization of proteins.
Further heterogeneity of ion channels arises when channels with different constitutive subunits give rise to a specific kind of current. Absence or mutation of one or more of the contributing types of channel subunits can result in loss of function and, potentially, underlie neurologic diseases.
Ion channels may be classified by gating, i.e. what opens and closes the channels. Voltage-gated ion channels open or close depending on the voltage gradient across the plasma membrane, while ligand-gated ion channels open or close depending on binding of ligands to the channel. Voltage-gated calcium channels: This family contains 10 members, though these members are known to co-assemble with α2δ, β, and γ subunits. These channels play an important role in both linking muscle excitation with contraction as well as neuronal excitation with transmitter release. The α subunits have an overall structural resemblance to those of the sodium channels and are equally large.
Calcium Channel Nomenclature
Calcium channels are generally classed as either high voltage-activated (HVA) or low voltage activated (LVA), depending on whether they open at more positive (e.g. −40mV) or more negative (e.g. −60 mV) membrane potentials, respectively (Figure 1). High voltage-activated channels can be further classified according to their pharmacological sensitivities and genetic α1 subunit protein (CaV) composition into L-type (CaV1.1-CaV1.4), P/Q-type (CaV2.1), N-type (CaV2.2) and R-type (CaV2.3). Low voltage-activated channels, also known as “T-type”, for their comparatively “tiny” or “transient” currents are further classified to according to their α1 subunit composition (CaV3.1-CaV3.3).Additional structural and functional variants of each CaV subtype can be generated by alternative splicing to produce a large number of different “splice variants” and therefore increase the repertoire and complexity of calcium channel properties. It is noted that CaV1.3 L-type and CaV2.3 R-type channels can exhibit characteristics of “mid-voltage-activated” channels, opening at membrane potentials that are more negative than HVA channels and more positive than LVA channels. For simplicity in this chapter, CaV3.1-CaV3.3 will be referred to as “T-type channels” and all other calcium channels will be referred to as “HVA channels”.
Figure 1:Voltage-gated calcium channels. (a) Schematic illustrating the topography of the high voltage-activated calcium channel complex showing the main pore-forming α1 subunit and ancillary subunits. The α1 and δ subunits are integral
Basic Functional Properties
Our present-day understanding of Ca2+ channels began with their electrophysiological isolation and description. Gating describes the opening and closing of channels. Typically, Ca2+ channels open (or activate) within one or a few milliseconds after the membrane is depolarized from rest, and close (deactivate) within a fraction of a millisecond following repolarization. Activation of Ca2+ channels is steeply voltage-dependent: channels open more quickly and with higher likelihood with larger depolarization’s. Inactivation, the closing of channels during maintained or repeated depolarization’s, strongly influences the cytosolic Ca2+ signal that arises from cellular electrical activity. While inactivation is a general property of Ca2+ channels, the speed of entry into and recovery from inactivation varies widely. 5 Bergsman, Wheeler, and Tsien. In addition to gating we consider two properties concerning the conduction of Ca2+ through the channel. Selectivity of voltage-gated Ca2+ channels for Ca2+ ions is remarkably high, so that Ca2+ is the main charge carrier even when Ca2+ is greatly outnumbered by other ions, as under normal physiological conditions. Permeation of Ca2+ through a single open Ca2+ channel can achieve rates of millions of ions per second when the electrochemical gradient is large. At driving forces reached physiologically, the flux rate is more modest, but sufficient to cause a large increase in [Ca2+]i(>1 μM) in a very localized domain (~1 μm) near the mouth of the open channel.
Calcium Channel Biophysical Properties
From their closed/resting state calcium channels open once the membrane potential depolarizes to a threshold point, at which the internal voltage sensor moves and the channel conformation changes to an open-pore calcium conducting state. Calcium channels only conduct ions in the open state and with ongoing depolarization an internal inactivation mechanism induces additional conformational changes to prevent further conduction. Once in the inactivated state, the channels can only be reopened by re-polarization to hyperpolarized membrane potentials, allowing the voltage sensor to return to its original closed conformation and the inactivation machinery to return to its de-inactivated position. Only from this state can further membrane depolarization reopen the channels to their ion conducting state. The membrane potentials and rates at which these steps occur varies between the calcium channel subtypes and splice variants, producing channel variants with widely differing conducting properties. [2, 4] Calcium channels are generally slower at opening (activation) and closing (deactivation) than typical voltage-activated sodium channels. Amongst the calcium channel subtypes, HVA channels generally display slower activation and faster deactivation that LVA channels. Further, HVA channels generally inactivate much more slowly than LVA channels. Together these properties result in HVA channels generating longer lasting calcium influxes upon sustained depolarization’s with T-type channels conducting more rapid and shorter calcium influxes under both brief and sustained depolarization’s (Figure 1). Of particular note, T-type channels also exhibit a distinct overlap of the membrane potentials at which they both activate and inactivate, uniquely enabling them to regulate sub-threshold excitability including mediating intrinsic oscillatory behavior’s and firing rates.
Molecular Physiology of Low-Voltage-Activated T-type Calcium Channels
Physiologic T-type Ca+2 channels were originally called low-voltage-activated (LVA) channels because they can be activated by small depolarization’s of the plasma membrane. In many neurons Ca+2influx through LVA channels triggers low-threshold spikes, which in turn triggers a burst of action potentials mediated by Na channels. Burst firing is thought to play an important role in the synchronized activity of the thalamus observed in absence epilepsy, but may also underlie a wider range of thalamocortical dysrhythmias. In addition to a pacemaker role, Ca+2entry via T-type channels can directly regulate intracellular Ca+2concentrations, which is an important second messenger for a variety of cellular processes.
Low-voltage-activated (T-type) calcium channels and autism
Overview
Low-voltage-activated (LVA) channels are characterized by threshold membrane potential for activation of macroscopic inward current of about –60 mV, which is below the threshold potential for action potential generation, and is far more negative than for the HVA channels. LVA channels open and inactivate very fast, but deactivate about 10 to 100 times slower than HVA channels. Single channel conductance of LVA channels is very low and is between 5 and 9 pS. For these reasons, they are also called T-type, T for transient (fast inactivation) and tiny (small conductance). LVA channels can be detected in various tissues such as heart, brain, dorsal root ganglia and adrenal gland.
The functional role of T-type channels in generating low-threshold spikes and rebound burst-firing has been demonstrated in neurons from the inferior olive, thalamus, hippocampus and neocortex.
Hydrophobicity analysis revealed that T-type channels – like HVA channels –contain four homologous repeats, each consisting of six trans-membrane segments (Figure 18). A comparison of the negatively charged residues in the pore loops shows that all T-type channels have a glutamate in repeats I and II and an aspartate in repeats III and IV (EEDD), whereas HVA channels have glutamates in all four repeats (EEEE). The positively charged residues of the HVA S4 voltage sensor are also conserved in the LVA channels (Figure 4). Overall amino acid sequences of the Cav3.1 and Cav3.2 channels exhibit 57% homology and their putative trans-membrane segments are 90% identical. The whole amino acid sequence of the Cav3.3 channel is 59.3% identical with the Cav3.1 sequence, and 56.9% identical with the Cav3.2 sequence. Its trans-membrane segments are only 80% identical with the trans-membrane segments of the Cav3.1 and Cav3.2 channels. Different splice variants of the Cav3.1 channel have been reported for the rat, mouse and human brain.
Voltage-dependent activation and inactivation of T-type calcium channels
Gating of T-type channels contrasts in many aspects the gating of L-type channels. When compared with L-type channels, the voltage dependence of T-type channel activation is shifted by 20–30 mV in the hyperpolarized direction, inactivation is rapid and not inherently voltage-dependent, and deactivation is slow. Parameters of voltage-dependent activation depend on the concentration of charge carrier used, but are not influenced by the choice of Ba2+ or Ca2+ ions. The rates of inactivation and recovery from inactivation of T-type channels are much faster than in any HVA channel. The time course of current decay during a single depolarizing pulse can be fitted by a single exponential.
Gating of T-type calcium channels
Steady-state activation of calcium current cannot be evaluated reliably from a current-voltage relationship. Analysis of voltage dependences of tail current amplitudes revealed two components of voltage dependence of activation of the T-type channel.
T-type channels and autism spectrum disorders
Autism spectrum disorders (ASDs) are a diverse group of neurological conditions characterized by impaired social interaction and communication with restricted and repetitive behavior. In a study examining the genetic components 461 patients with ASDs, mutations were identified in theCACNA1Hgene encoding CaV3.2 in six patients from different families (Fig. 1) [4]. While loss-of-function effects have been observed as a result of the mutations introduced into exogenously expressed CaV3.2 channels, the low incidence of occurrence and lack of segregation with the disease phenotype suggest that any role in ASD is likely part of a much wider neural impairment. Of note, a recent study has identified theCACNA1Ggene as a potential novel candidate for ASD [5].
Fig. 1. Calcium channel structure and human mutations. (a) Schematic of the HVA calcium channel complex indicating the pore forming a1 (Cav) subunit along with ancillaryb, c, and a2dsubunits. (b) Predicted topology of thea1 subunit with its four domains (I-IV) and six Trans membrane segment (S1-S6) structure. Inset (colored dots) Locations of known human calcium channel mutations according to domain and segment (multiple mutations in same segment or linker are represented with a single dot); Childhood Absence Epilepsy and Idiopathic Generalized Epilepsy Mutations (Epilepsy), Autism Spectrum Disorders (ASDs), Familial Hemiplegic Migraine type-1 (FHM1), Episodic Ataxia type-2 (EA2), Spinocerebellar Ataxia type-6 (SCA6), Incomplete X-linked Congenital Stationary Night Blindness (IXLCSNB), X-linked Cone-Rod Dystrophy (CORDX), Hypokalemic Periodic Paralysis (HypoPP), Malignant Hyperthermia Susceptibility (MHS), Timothy Syndrome, Brugada syndrome, Sino-Atrial Node Dysfunction and Deafness (SANDD). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Computational modeling of autism
Overview
Autism manifests during the first three years of life. The core features of autism [31] are qualitatively impaired socialization, impaired verbal and nonverbal communication, and restricted and repetitive patterns of behavior, interests, and activities. Children with autism are impaired in at least one or more of the following areas with onset before three years of age: social interaction; language as used in social communication; and symbolic or imaginative play.
Autism includes a spectrum, or broad range, of behavioral and cognitive manifestations. Mental retardation is common but between one fourth and one third of autistic individuals have IQs in the normal range or above [24]. Approximately one third of autistic individuals develop epilepsy by adult-hood . Twenty to thirty percent of individuals with autism have apparent regression of skills after a period of apparent normal development, typically between one and three years of age .
The term Autistic Spectrum Disorder (ASD) refers to this entire range of functions and findings within those individuals who have the key triad of impairments in social interaction, verbal and non-verbal communication, and restricted, repetitive, and stereotyped interests and activities. This term includes individuals who range from low-function, with profound impairments in many areas, to individuals with high-function. The Diagnostic and Statistical Manual of Mental Disorders defines autism as part of the group of Pervasive Developmental Disorders. Both Autistic Disorder and Pervasive Developmental Disorder Otherwise Specified/Atypical Autism (PDD-NOS) are generally considered part of autism. Asperger Syndrome shares the core deficits in social interactions and restricted/repetitive behaviors and interests, while relatively sparing formal language development. Asperger Syndrome individuals nevertheless have abnormal social communication skills. The term “autistic spectrum” was coined to include those diagnostic labels within the DSM IV and the International Classification of Diseases and other individuals with the key features of impairments in social interaction, impairments in verbal and non-verbal communication with a narrow/repetitive pattern of behaviors, and with impairments in imagination. Wing asserted that the extant diagnostic subdivisions were “arbitrary and … difficult to apply and unhelpful in clinical practice.” Wing instead subdivided the spectrum by the nature of the social impairment and related co-morbidities: “aloof”; “passive”; “active but odd”; and “loners.” While those subdivisions have not found broad usage, the concept of an autistic spectrum has found common if not universal acceptance. Many researchers and clinicians accept the concept, although some caution that it implies stronger links between the disorders than current research may justify. Also its common use may have led to excessive clinical labeling of some socially atypical children as autistic. The goal of this paper is to understand how the range and common themes of the entire heterogeneous autistic spectrum, which will henceforth be referred to as “autism,” can result from various combinations of disruptions in an established model of normal brain function. The model does this by proposing how breakdowns of multiple mechanisms in several brain areas may be involved in the generation of autistic behaviors. Differences ion the degree of malfunction across these mechanisms may help to account for the differences in autistic populations. To accomplish this task we must first establish what clinical phenomena an adequate model of autism must explain.
An early manifestation of autism is a failure to develop basic imitation skills. Normal children usually show basic imitative behaviors by the end of the first year, and are imitating complex actions like wiping a table by fifteen months. This is not so of most autistic children.
The communication deficits of autism often have onset before spoken language typically begins. Preverbal communication by way of gestures, sounds, and expressions are deficient. Many children with autism stay essentially non-verbal. Among those autistic individuals who develop language, other communication deficits follow and are not mere delays of the normal pattern of development, but are instead wide-ranging and complex disorders. Language pragmatics is often disturbed. Inappropriate and idiosyncratic word usages, such as inappropriate generalizations of meaning and odd analogies, are common. Often, individual words may be used with hyper specificity and without ever being able to apply the word to a more general concept. Unusual intonation, echolalia, and pronoun reversal are common.
Extreme unevenness in cognitive skills is a common feature of autism. Some autistic individuals have “islands” of normal or occasionally even superior ability and a few have narrow skill sets that are so superior to normal populations that they are referred to as “savants”. These areas of higher ability often include mathematical and musical skills. Autistic individuals also have facilitated skills at detecting hidden, embedded figures and at copying “impossible figures”. Thus, the autistic cognitive style is notable for its extreme concreteness, with autistic individuals doing relatively well on tasks that require rote memory but characteristically poorly on tasks that require higher-4 order conceptual processes or abstraction. By eighteen months, an autistic child often lacks the normally emergent imaginary or symbolic play. Instead, an autistic child may tenaciously perseverate on specific features of a toy. Moreover in children with these tendencies, a favorite object must be exactly right (i.e., how it was when it was first noticed) and is often played with according to a very specific routine. It is as if each situation is learned as a complete specific whole and any variation from that standard invalidates any understanding that an autistic has of the situation and what to expect. When this “need for sameness” is violated by even minor variations in routines, behavioral compensation in the form of strong emotional outbursts may result.
Extreme negative emotional reactivity is not only triggered by variations in routines; basic sensory stimuli, such as noise, smells, or light touch, can be emotional triggers for many as well. This contrasts dramatically with hypo-responsiveness to other, and in particular, social stimuli, such as one’s name being called, facial reactions, or praise.
Attentional differences are prevalent among patients with autism and include deficient “shared” or “joint” attention. Shared attention, which usually emerges during a normal child’s first year of life, and refers to the ability to follow a significant other’s gaze and thus to share attention in external objects with others, is characteristically deficient among those with autism. Reviews of home movies have documented attentional differences, with deficient attention in social but not in non-social areas, before six months of age in infants who were later to be identified as autistic. Autistic individuals also commonly experience difficulties with disengaging or shifting attention and with splitting attention between objects. Paradoxically, some autistic individuals clinically appear to have difficulty sustaining attention in certain contexts and some studies have documented a significant frequency of symptoms severe enough to warrant labeling this behavior as comorbid Attention Deficit Disorder in some autistic spectrum individuals.
Individuals with autism, especially low functioning individuals with autism, are also prone to repetitive stereotypic movements, such as rocking, hand flapping, and head banging. Other motor abnormalities variably include poor motor imitation abilities, generalized clumsiness, and gait abnormalities. High functioning individuals with autism tend to have large handwriting even when controlled for educational level. A retrospective review of videotapes of infants later diagnosed to have autism suggested that movement abnormalities may be the earliest behavioral feature in autism, with abnormalities present by four to five months of age.
Brain abnormalities cause autism
Various sorts of analysis have been employed to attempt to determine what brain abnormalities cause autism. These analyses have included cyto-architectural studies, imaging of structural volumes, functional imaging studies, genetic and gene expression studies, lesion studies, and studies using behavioral paradigms known to be associated with particular brain structures.
Systems implicated have included the cerebellar limbic and neocortical systems. The iSTART model clarifies how the various abnormalities that are summarized below all contribute to the creation of autistic behavioral symptoms via the interaction of different types of functional deficits across these brain areas.
Computational Modeling Overview
One of the main goals of neuroscience research is the understanding of the functional rules governing the behavior of microcircuits in the vertebrate nervous system. Recent advances in cell and organotypic neuronal cultures allow the experimenter to create in vitro net-works of neurons that represent rudimentary approximations of such microcircuits. Moreover, the electrophysiological technique based on the use of planar microelectrode arrays can be used to characterize the dynamics of such networks. As a result, nowadays the neuro science community has access to an increasing amount of long term multi channel recordings, obtained by in vitro coup-ling networks of neurons to arrays of substrate planar microelectrodes. Elementary neuro-computational rules are hidden inside these long-term multichannel recordings and a powerful tool to decipher them is provided by the extensive use of simulated experiments, along a scientific tradition already well developed in several branches of physics. Specific simulated experiments will be described in the following: first, a detailed model of single and synoptically connected neurons will be described, appropriate computer simulate the action potentials of neuronal populations. Then ‘realistic’ signals will be generated. These signals are intended to reproduce, both in shape and intensity, those recorded by a microelectrode array. Typical experimental conditions will be considered and a detailed analysis will be given, of the bioelectronics coupling and of its influence on the shape of the recorded signals.
Computational Model:
A computational model is a computer program, run on a single computer, or a network of computers, that attempts to simulate an abstract model of a particular system. Computer simulations have become a useful part of mathematical modeling of many natural systems in physics (computational physics), astrophysics, chemistry and biology, human systems in economics, psychology, social science, and engineering. Simulation of a system is represented as the running of the system’s model. It can be used to explore and gain new insights into new technology, and to estimate the performance of systems too complex for analytical solutions.
Computational Modeling and Simulation
In engineering practice there is always a pair of model – a real system. From this perspective, models are divided into two groups:
• Models, which allow you to analyze a real system. They allow specifying and clarifying our ideas of an existing system.
• Models resulting from development and design (if the task concerns the synthesis of design and construction). This activity is usually supported by computer technology. Thus we speak about methods of design automation, construction, manufacturing etc. For example, CAD, CAP, CAM, etc. Each scientific and technical discipline has its specific problems in creation of models. It should always be based on thorough (physical, chemical, biological, social etc.) knowledge of the concerned branch of business. In our approach we will emphasize in particular that the ultimate goal is to implement models using computer aided equipment or computers. The computer thus enables to implement full or partial model showing the system studied or projected. Therefore the concept of a computer model or computer-aided system. Process of experimentation with the model is called simulation. Simulation experiments allow the search for alternatives and appropriate parameters of the projection system, eventually allow correction or completion of knowledge of the analyzed system.
Discrete and Continuous Simulation
The behavior of multidisciplinary systems is a combination of continuous time physical phenomena and events occurring at discrete space and time coordinate. For high-fidelity simulation of such systems, hybrid modeling and simulation is required in which both continuous and discrete event phenomena can be represented. Many physical phenomena, such as rigid body motion, flow of electric currents, fluid flow, or heat flow, evolve as continuous functions of time and are therefore best modeled by a set of differential algebraic equations (DAEs) .Physical events and digital components, on the other hand, generate outputs at discrete points in time and space; they are best modeled using discrete variables or impulse functions. Examples include rigid body collisions, data buses, and digital controllers. In addition, discrete event simulation is applied to a variety of other disciplines, including logistics, transportation, material handling, and military simulation. A good overview of the principles and industrial applications of discrete-event simulation can be found in. Because mechatronic systems combine both continuous time phenomena and discrete events, they require mixed continuous-discrete models. Several simulation languages, including Model ica and VHDL-AMS, support mixed systems modeling. These models also require advanced solvers that efficiently synchronize between DAE solving and discrete event propagation. Most commercial simulation software packages include this capability now.
Simulation Types
Computer models can be classified according to several independent pairs of attributes, including:
- Stochastic or deterministic (and as a special case of deterministic, chaotic) – see External links below for examples of stochastic vs. deterministic simulations
- Steady-state or dynamic
- Continuous or discrete (and as an important special case of discrete, discrete event or DE models)
- Local or distributed.
Another way of categorizing models is to look at the underlying data structures. For time-stepped simulations, there are two main classes:
- Simulations which store their data in regular grids and require only next-neighbor access are called stencil codes. Many CFD applications belong to this category.
- If the underlying graph is not a regular grid, the model may belong to the mesh free method class.
Equations define the relationships between elements of the modeled system and attempt to find a state in which the system is in equilibrium. Such models are often used in simulating physical systems, as a simpler modeling case before dynamic simulation is attempted.
- Dynamic simulations model changes in a system in response to (usually changing) input signals.
- Stochastic models use random number generators to model chance or random events;
- A discrete event simulation (DES) manages events in time. Most computer, logic-test and fault-tree simulations are of this type. In this type of simulation, the simulator maintains a queue of events sorted by the simulated time they should occur. The simulator reads the queue and triggers new events as each event is processed. It is not important to execute the simulation in real time. It’s often more important to be able to access the data produced by the simulation, to discover logic defects in the design, or the sequence of events.
- A continuous dynamic simulation performs numerical solution of differential-algebraic equations or differential equations (either partial or ordinary). Periodically, the simulation program solves all the equations, and uses the numbers to change the state and output of the simulation. Applications include flight simulators, construction and management simulation games, chemical process modeling, and simulations of electrical circuits. Originally, these kinds of simulations were actually implemented on analog computers, where the differential equations could be represented directly by various electrical components such as op-amps. By the late 1980s, however, most “analog” simulations were run on conventional digital computers that emulate the behavior of an analog computer.
- A special type of discrete simulation that does not rely on a model with an underlying equation, but can nonetheless be represented formally, is agent-based simulation. In agent-based simulation, the individual entities (such as molecules, cells, trees or consumers) in the model are represented directly (rather than by their density or concentration) and possess an internal state and set of behaviors or rules that determine how the agent’s state is updated from one time-step to the next.
- Distributed models run on a network of interconnected computers, possibly through the Internet. Simulations dispersed across multiple host computers like this are often referred to as “distributed simulations”. There are several standards for distributed simulation, including Aggregate Level Simulation Protocol (ALSP), Distributed Interactive Simulation (DIS), the High Level Architecture (simulation) (HLA) and the Test and Training Enabling Architecture (TENA).
Methodological approach to modeling and simulation
Definition of the problem specification (implemented after consultation with experts in the subject field) implies:
• Definition of dynamic variables entering the balance of considerations,
• Indication of inputs, outputs and states of the model,
• The structure of model (links determination),
• Model relevance definition and the limits for each variable.
In the design stage of the model development process, the experimentation strategy for models and its
Systematic expressions are defined. Diagrams are the product of the process. In the model representation stage a decision on how to program the model is made, while taking into Consideration programming language, data views and their inputs and outputs.
The model behavior verification stage is a quality stage to evaluate the trends of dynamic variables, their dependence on initial conditions, the validity of the limited space coordinates etc. Definition of the problem
In the phase of calculations (experimentation) on models calculations are made, followed by the result printing, optionally with their graphics processing. Variable courses are being tested. The verification is done by software testing and control examples.
Comparison of models and real object (system) correlation is performed in the stage of decision on the success and new knowledge gaining. Respective/proper experimentation with the model (simulation) takes place which results in the new knowledge acquisition.
Aim of modeling and simulation
Modeling and simulation technique depends on:
• Development, which as a separate science and engineering disciplines,
• Development of mathematical methods,
• Progress in those disciplines in which the models are formulated,
• Development of new technical, particularly computer equipment,
• Development of programming language resources.
Virtually all areas of modeling can be focus to the objectives and the means defining projects computer systems.
Methods and results
Introduction
Human brain consists of billions of interconnected neurons. Brain is the most complex and delicate organ of the human body in which the forebrain is the largest part of the brain which consists of the cerebrum, the thalamus and the hypothalamus.
Various researchers indicate that rat subthalamic projection nucleus neuron are equivalent to human nucleus. All of these neurons have basically the same morphology, a cell body with dendritic trunks with complex branching. To model subthalamic nucleus neuron the following procedures are followed:
- Create a single compartment neuron model with Hodgkin-Huxley conductance’s, run the simulation and displaying the simulation results.
- Building multi-compartmental neurons and using different types of graphs to display the results.
- Replicate neurons using templates and connect these neurons together.
- Add new membrane mechanisms to the simulator and incorporate them in this neurons using NMODL
Creating a soma
In a cell’s soma (and dendrites), are represented by what are called sections. Sections in are cylindrical in nature and, for computational reasons, can be divided in to smaller areas called segments. Our soma is approximately spherical with a diameter of 18.8µm.The syntax for creating this section is as follows:
Create soma
This command creates a new section with default properties
- number of segments in the section (soma.nseg = 1)
- diameter (soma.diam = 500 µm)
- length (soma.L =100 µm)
- specific membrane capacitance [1 µF/cm2]
- cytoplasmic resistivity (Ra = 35.4 ohm-cm)
6.2.1 Changing the default parameters
For our simulation, we need to change some of our soma’s properties; the diameter of the section (diam), the length of the section (L), and the axial resistance (Ra), which in the rat subthalamic nucleus has a high value of 123 ohm-cm. All of these values are taken from data specific to subthalamic projection s.
Group multiple properties within braces:
Soma{nseg = 1 diam = 18.8 L = 18.8 Ra = 123.0}
Inserting membrane channels (mechanisms) throughout the segment
Each section created in this fashion has the default properties automatically inserted, but other mechanisms (e.g., channels) with their own properties must be explicitly inserted into a section. Includes two built-in channel membrane mechanisms: Hodgkin-Huxley channels (hh) and passive channels (pas). Each of these mechanisms can be inserted using the insert command.
Code:
Soma insert hh
soma insert pas
Now add the new membrane mechanism’s properties and their default values to the section.
- g_pas (specific membrane conductance [S/cm2]) and
- e_pas (reversal potential [mV]).
For our simulation, neither the default Hodgkin-Huxley channels nor the passive properties will accurately model type. However, for the moment we will leave the default properties to explore a working system.
The Hodgkin-Huxley channels add the following new properties to the section:
- gnabar_hh: The maximum specific sodium channel conductance [Default value = 0.120 S/cm2]
- gkbar_hh: The maximum specific potassium channel conductance [Default value = 0.036 S/cm2]
- gl_hh: The maximum specific leakage conductance [Default value = 0.0003 S/cm2]
- ena: The reversal potential for the sodium channel [Default value = 50 mV]
- ek: The reversal potential for the potassium channel [Default value = -77 mV]
- el_hh: The reversal potential for the leakage channel [Default value = -54.3 mV]
It also adds the following state variables that can be displayed with the print command:
- m_hh: The sodium activation state variable
- h_hh: The sodium inactivation state variable
- n_hh: The potassium activation state variable
- ina: The sodium current
- ik: The potassium current
Adding an electrode (point process)
Makes the distinction between mechanisms that are attributed to an entire section (e.g., HH channels) and mechanisms that are associated with a particular point in the section (e.g., voltage clamp electrode or synapse). While the former are most conveniently expressed in terms of per unit area, the point processes are more conveniently expressed in absolute terms (e.g., current injection is usually expressed in terms of nA instead of nA/cm2).
Point processes are handled as objects which mean that to create one object variable to be associated with the object and then create a new object. To declare object variables, enter the following line:
Objectvar electrode
This creates an object variable named electrode.
Newly created objects need to be associated with a particular section, so it need either to have a default section or to specify the section name with which the object is to be associated.
As with channels, each point process has its own set of properties. Below is a list of IClamp point processes properties.
IClamp:
- del: The delay until the onset of the stimulus (in ms)
- dur: The duration of the stimulus (in ms)
- amp: The amplitude of the stimulus (in nA)
There are several built-in point processes, including: IClamp, VClamp and ExpSyn.
Set properties of electrode
In our model, we want to stimulate the soma by giving it a current pulse. It is accomplish by adding setting the properties of the IClamp.
To set the properties of point processes we must use the dot notation:
somaelectrode.del= 100
soma electrode.dur = 100
soma electrode.amp = 0.45
This creates a current clamp electrode in the centre of the soma. This electrode will start injecting a 0.4nA current pulse 100ms after the start of the simulation. Therefor a duration of pulse will be 100ms after which it will turn off.
Running the simulation
Before running the simulation it needs to be told how long to run. As the default, simulations run for 5 ms of simulated time, but this can be changed by setting the tstop variable to another value (in ms). For example, to run a simulation for 300ms, enter:
tstop = 300
This will set the simulation stop time to 300ms and run the simulation from the beginning.
After running the simulation types it in the console window.
Print soma.v
This line tells the program to display the membrane potential of the soma after running the simulation for 300 msec
Adding the display of all variables to this program
To display properties and the current values of the sections in this model, call the psection function. To see the properties of a specific section:
- psection() – to list the properties of the default section defined by the access command
- soma psection() – to list the properties of a particular section in the model:
- forallpsection() – to list the properties of all sections in the model:
Add the forallpsection () statement on the line after print soma.
Avoiding tedious typing: for loops
passive channels are inserted and set their properties for dend[0] and dend[1] individually. This is OK when there are only two dendrites, but what if there were 100 dendrites. It will need to type out 100 times three lines or do lots of cutting and pasting; boring and liable to lead to repetitive stress syndrome (RSI).
The solution is the for loop. A for loop can step through each element in an array in turn. The format of the for loop is:
forvar=lower-bound, upper-boundcommand
Wherever is the loop variable, lower-bound is the lower bound of the loop, upper-bound is the upper bound of the for loop and command is the command to run through the loop
This statement doesn’t replace the whole line which defines dend[1] and dend[0]. It only substitutes for the parts that are consistent across all dendrites.
The arrays, of which the dendrites are defined, are indexed by starting with the number zero not 1. This is why the for loop begins with zero and goes to the number of dendrites minus 1.
Create a realistic model
Now dramatically enhance this neuron by introducing a more complete dendritic tree morphology that will be read from two files (one for each of the two trees).The system for adding the realistic morphology is similar to that used in creating the two dendrites (each being 1 section, 5 segments) there are two dendritic trees, containing 23 (defined in treeA.dat) and 11 (defined in treeB.dat) sections respectively .To access a file, create a new file object. This is done in a similar manner to creating other objects
Viewing the results
If we open a shape plot (Graphs>>Shape) and run the program again, we should see the graph. To see the color version, as displayed to the left, we will need to right-click on the image and select “Shape Plot” from the menu. Otherwise, it will simply be in black and white. Also, after changing to a shape plot, adjust the thickness of the branches by right-clicking and selecting “Show Diam:” from the Shape Style option.
The final, with full dendritic tree morphology is shown here in a shape plot. This form of shape plot shows the voltage of the sections as a hot scale (hot colors mean high voltage, cool colors low voltage as indicated in the scale). By opening the run control window (Tools>>Run Control) and adjusting the Int (mV) value, you can change the color of the cell.
In three dimensions it is sometimes difficult to see the real extent of the dendritic arborization. In this case it helps to rotate the image. To do so right-clickon the image and select “3D Rotate”. Then use the left button to rotate and the combination of cntl-left click to move the image.
in this example, each branch/section only has one segment, independent of how long the branch may be. You may want to increase the number of segments for a higher spatial resolution
Creation of channels
To make this neurons much more electrophysiologically characteristic of subthalamic nucleus neurons. There are many channel types exists.
An important electrophysiological feature of subthalamic neurons is the post hyperpolarising response. When a neuron is hyperpolarised (e.g. by current injection or inhibitory synaptic input), at the end of the hyperpolarisation, a burst of activity is observed in subthalamic projection neurons. This response is mediated by a low threshold calcium selective ion channel, called the T-type calcium channel.
The current IT produced from the T-type calcium channels was characterized within the Hodgkin-Huxley framework by Wang et al. (1991):
wheregT (max) is the maximum T-type calcium conductance, r is the activation state variable, s is the inactivation state variable, ECa is the reversal potential for calcium and V is the neuron membrane potential.
The state variables r and s are given by the following kinetic systems:
Here “C” refers to a closed state and “O” refers to an open state. The α and β variables are the forward and backward rate constants from one state to another; they are voltage dependent functions specified in Wang et al. (1991). Note that inactivation is a three state kinetic process, with fast (s) and slow (d) components).
The kinetic schemes translate to three differential equations:
Instead to learn the Model Description Language (NMODL) provided for defining additional distributed membrane mechanisms such as ion channels and calcium pumps and point processes such as synapses.
NMODL
A description of a membrane mechanism in NMODL is a text file divided into a number of blocks. A block begins with a keyword defining the type of block, then an open brace “{“, followed by block specific definitions, and finally, the block is ended with a closing brace “}“.
There are a number of NMODL examples provided with the NEURON package. Here we will go through, step by step, an example of a new channel mechanism.
The Title and Unit Block:
We start the NMODL file with some standard definitions useful in most channel membrane mechanisms:
TITLE Calcium T channel for Subthalamic Nucleus
UNITS {
(mV) = (millivolt)
(mA) = (milliamp)
- The TITLE keyword allows us to identify what this file is describing, though it is not required.
The NEURON block
The NEURON block is the public interface of the mechanism. It tells the hoc interpreter how to refer to the mechanism and what variables it can see or change. The structure of the block is as follows:
NEURON {
SUFFIX suffix
USEION ions… READ vars… WRITE vars…
RANGE var, var,…
GLOBAL var,var,…
The first step is to identify this mechanism from all other membrane mechanisms when referencing it from the hoc language. This is done through the SUFFIX statement of the block. Access to all variables in this mechanism from the hoc file is then done using the suffix. this channel mechanism “CaT”, so to access variables in the mechanism from hoc we use var_CaT (where var is a variable in this mechanism).
The USEION statement specifies what ions this channel mechanism uses. There are three ions NEURON knows about, na, k, ca, however, others may also be defined via this statement. NEURON can keep track of the intracellular and extracellular concentrations of each ion. Dealing with ions is difficult, because more than one mechanism may affect a particular ion Therefore, when dealing with ions use exactly the same name used in all other mechanisms. The READ modifier lists ionic variables needed in calculating the ionic channel current (usually the equilibrium potential, or concentration). The WRITE modifier lists what ionic variables are calculated in this mechanism (usually the current). In this we use:
USEION ca READ eca WRITE ica
Eca is the equilibrium potential for ion ca, and ica is the calcium current, to be calculated in this mechanism.
The RANGE statement makes the following variables visible to the NEURON interpreter and specifies that they are be functions of position. For example, the maximum channel conductance should be a RANGE variable, since it can be different at different points on a neuron.
The GLOBAL statement specifies variables that are always the same for the mechanism. This mechanism does not have any GLOBAL variables. Final NEURON block now has the form:
NEURON {
SUFFIX CaT
USEION ca READ eca WRITE ica
RANGE gmax
The PARAMETER block:
The PARAMETER block specifies variables that:
- are not changed as a result of the calculations in the mechanism;
- are (generally) constant throughout time; and
- Can be changed by the user from the hoc interface or the GUI.
From the equation:
That in calculating this channel current this model will use the voltage v, the maximum conductance declared in the NEURON block above, gmax and the calcium equilibrium potential, eca. Of these only gmax satisfies the conditions above; v is calculated at every time step by NEURON rather than being specified by the user, and eca may also be calculated at each time step depending on the calcium concentrations.
The ASSIGNED block:
The ASSIGNED block declares variables that are either:
- calculated by the mechanism itself or
- Computed by NEURON.
Variables that this mechanism will compute are the calcium current ica, and variables for the rate equations ralpha, rbeta, salpha etc. The variables that the mechanism uses that are computed by NEURON are the membrane potential v and the calcium equilibrium potential eca.
The STATE block:
The STATE block declares state variables. One type of state variable are the variables that are trying to solve for in kinetic schemes. There are three state variables in this kinetic channel model, r, s and d.
The heart of the mechanism:
Now come to the heart of the mechanism. To calculate the values of the variables r, s and d in order to calculate the calcium current from the above equation. These are given by the three kinetic differential equations:
NMODL and NEURON:
A membrane mechanism description using NMODL is laid out in a text file. The NEURON interpreter cannot read this file directly as it can with hoc files. Instead, the NMODL file has to be compiled into a form that NEURON can use. Creating a file CaT.mod containing our description of the T-type calcium channel in NMODL.
At the OS prompt in the directory that contains i386. SthD.hoc is the file that contains this model specification, and it is nearly identical to sthC3.hoc from tutorial part C.
MSWin users:
MSWin users should just launch
Start / Programs / Neuron / mknrndll
This brings up a directory browser that can be used to navigate to the directory that contains the CaT.mod file. On the proper directory, click on the button labelled “Make nrnmech.dll”. This compiles all the mod files in this directory and creates a file called nrnmech.dll that contains the new compiled mechanisms. nrnmech.dll will be automatically loaded when we double click on a hoc file in this directory.
Membrane mechanisms are used on every time step, and therefore need to be efficient. Converting the NMODL file to C-code and then compiling this into a new NEURON program or library (which is what nrnivmodl and mknrndll do) leads to more efficient simulation.
An important electrophysiological feature of subthalamic neurons is the post hyperpolarizing response. When a neuron is hyperpolarized (e.g. by current injection or inhibitory synaptic input), at the end of the hyperpolarization, a burst of activity is observed in subthalamic projection neurons. This response is mediated by a low threshold calcium selective ion channel, called the T-type calcium channel.
The current IT produced from the T-type calcium channels was characterized within the Hodgkin-Huxley framework by Wang et al. (1991):
Now injecting a hyperpolarizing current in one of our neurons, we now observe a post-hyperpolarizing T-type response:
Data used to generate the T-type response shown below:
Soma properties:
| Nseg | diam | L | Ra | gnabar_hh | gl_hh | el_hh |
| 1 | 18.8 | 18.8 | 123.0 | 0.25 | .0001666 | -60.0 |
Discussion and conclusion
Autism is a neural developmental disorder characterized by impaired social interaction and communications, and by restricted and repetitive behavior. Autism affects information processing in the neuron of the brain by altering the connections of the neuron and their synapse
Human brain consists of billions of interconnected neurons. Brain is the most complex and delicate organ of the human body in which the forebrain is the largest part of the brain which consists of the cerebrum, the thalamus and the hypothalamus.
A single compartmental neuron is created by incorporating soma, membrane channel and electrode properties to the neuron. In this program the soma is the default section so the soma voltage is plotted by default. After the simulation a voltage graph display the output voltage in the soma. Using the psection() function the current values of the sections in this model are displayed.
Adding more section representing the two dendrites make this single compartmental neuron to multi-compartmental. Electric potential travelling down the dendrites to the soma implying that spatial dimensions are important. Increasing the number of segments in the section increase the spatial resolution of the dendrite. This is very important for accurate simulation and this has the effect of increasing the spatial resolution of the cable equation. This model is entirely independent of the numerical details because the reason for distinction between sections and segments allow to create models that do not rely on the number of segments in the model.
Normally the electrical potentials travelling down the dendrites to the soma implying that spatial dimensions are important. Increasing the number of segments in the section are increase the spatial resolution of the dendrite. The two dendrites contain 23 and 11 sections respectively for creating a realistic model neuron.A voltage graph are plotted the voltage halfway down the default section and it is done for comparing the voltage of the soma and a distal part of a dendrite.A model description language (NMODL) is provided for defining additional distributed membrane mechanisms such as ion channels and calcium pumps and point processes such as synapses to make the neurons much more electrophysiological characteristic of subthalamic nucleus neurons.Finally after the simulation a post hyperpolarizing T-type response graph is plotted by injecting current in any neuron.
Various researchers indicate misses’ mutations in the calcium channel in 6 of 461 individuals with ASD, which does not produce post hyperpolarizing T-type response. That means only autistic brain neuron produce the T-type response. Functional analysis shows that all these mutations significantly reduce calcium channel activity and thus could affect neuronal function and potentially brain development.
Till today, there is no core mechanism that could explain the assortment of symptoms found in autism. In fact, autism is a mysterious brain disorder in which numerous abnormalities in the activity of brains prevail. Thus the absence of detectable level of T-type currents in dissociated STN neurons may be attributable to loss of dendritic processes during dissociation, and suggests the possibility that STN neurons express T-type channels preferentially in dendrites[32]. The function of the STN is unknown[29], but current theories place it as a component of the basal ganglia control system that may perform action selection. This notion, however, is based on the negative finding of T-type currents in dissociated neurons.
To explore the exact biological cause of autism, molecular investigations of the anatomical and functional connectivity of neuron are very important which need further detailed study.
References
[1] American Psychiatric Association, 1994. Diagnostic and statistical manual
of mental disorders, fourth ed. (DSM-IV). American Psychiatric
Association, Washington, DC.
[2] Volkmar, F., Lord, C., Bailey, A., Schultz, R.T., Klin, A., 2004. Autism
and pervasive developmental disorders. J. Child Psychol. Psychiatry 45
(1), 135–170.
[3] Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell
P.Recognition of mental state terms. Clinical findings in children with
autism and a functional neuroimaging study of normal adults. Br J
Psychiatry 1994; 165: 640–9.
[4] Splawski, I., Yoo, D. S., Stotz, S. C., Cherry, A., Clapham, D. E., and
Keating, M. T. (2006) CACNA1H mutations in autism spectrum disor-ders.JBiolChem 281, 22085–22091.
[5] Strom, S. P., Stone, J. L., Ten Bosch, J. R., Merriman, B., Cantor, R. M.,
Geschwind, D. H., and Nelson, S. F. (2010) High-density SNP associa-tion study of the 17q21 chromosomal region linked to autism identi-fies CACNA1G as a novel candidate gene. Mol Psychiatr15, 996–1005.
[6] Hines, M. L. and Carnevale, N. T. (2000) “Expanding NEURON’s repertoire of mechanisms with NMODL” Neural Computation12: 839-851
[7] Johnston, D. and Wu, S. (1995) Foundations of Cellular Neurophysiology. MIT Press, Cambridge, Massachusetts.
[8] Wang, X., Rinzel, J. and Rogawski, M. (1992) “A model of the T–type calcium current and the low threshold spike in thalamic neurons”. J. Neurophys. 66: 839-850
[9]Wade, Nicholas (1999-10-15). “BRAIN MAY GROW NEW CELLS DAILY”. The New York Times.
[10] http://www.pnas.org/content/103/33/12219.full at 25-12-2012
[11] Davies, Melissa (2002-04-09). “The Neuron: size comparison”. Neuroscience: A journey through the brain. Retrieved 2009-06-20.
[12] Chudler, Eric H..”Brain Facts and Figures”.Neuroscience for Kids.Retrieved 2009-06-20.
[13] Herrup K, Yang Y (Ma3371545712287 Content-Dispositin2124 2007). “Cell cycle regulation in the postmitotic neuron: oxymoron or new biology?”.Nat. Rev. Neurosci.8 (5): 368–78. doi:10.1038/nrn2124. PMID 17453017.
[14] Alvarez-Buylla A, Garcia-Verdugo JM (February 1, 2002). “Neurogenesis in adult subventricularzone”.Journal of Neuroscience22 (3): 629–34. PMID 11826091.Retrieved 2009-06-20.
[15] Parent, A; Carpenter MB (1995). “Ch. 1”. Carpenter’s Human Neuroanatomy.Williams&Wilkins.ISBN 978-0-683-06752-1.
[16] Cosgrove, KP; Mazure CM, Staley JK (2007).”Evolving knowledge of sex differences in brain structure, function, and chemistry”.BiolPsychiat62: 847–55. PMC 2711771.PMID 17544382.
[17] C. Davison Ankney (1992). “Sex differences in relative brain size: The mismeasure of woman, too?”.Intelligence16 (3-4): 329–336. doi:10.1016/0160-2896(92)90013-H.
[18] Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE (1999). “Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance”. The Journal of Neuroscience19: 4065–72. PMID 10234034.
[19] Kandel, ER; Schwartz JH, Jessel TM (2000).Principles of Neural Science.McGraw-Hill Professional.p. 324.ISBN 978-0-8385-7701-1.
[20] Book- neuroscience and behavior.
[21] Book- Brain Facts, a primer on the brain and nervous system by Society for Neuroscience.
[22] Birnbaumer L., Campbell K. P., CatterallW. A., Harpold M. M., Hofmann F., HorneW.
A., Mori Y., Schwartz A., Snutch T. P., Tanabe T., et al. (1994): The naming of voltage-gated calcium channels. Neuron 13, 505—506
[23] Mirror neuron system and autism – MartinosCenter for Biomedical by N Hadjikhani.
[24] Mirror neuron system and autism by N Hadjikhani.
[25] WHO NTD Report 2010
[26] Keiser J and Utzinger J (2010) “The drugs we have and the drugs we need
against major helminth infections.” Advances in Parasitology 73: 197-229.
[27] Raymond V and Sattelle DB (2002) “Novel animal-health drug targets from ligand-gated chloride channels.” Nature Reviews: Drug Discovery 1: 427-436.
[28] Geary TG (2005) “Ivermectin 20 years on: maturation of a wonder drug.” TRENDS in Parasitology 21: 530-532.
[29] Hu Y et al. (2009) The New Anthelmintic Tribendimidine is an L-type (Levamisole and Pyrantel) Nicotinic Acetylcholine Receptor Agonist. PLoS NTD 3: e499.
[30] Doenhoff MJ et al. (2008) “Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis.” CurrOpin Infect Dis 21: 659-667.
[31] American Psychiatric Association, 1994