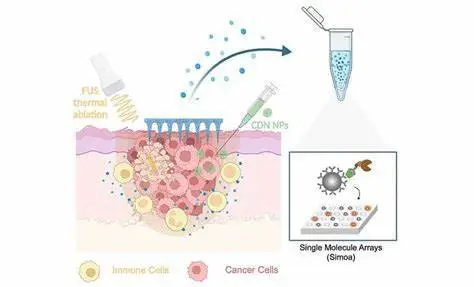
Skin cancer monitoring is an important part of early detection and treatment. A team of researchers from Harvard University’s Wyss Institute, MIT, and Brigham and Women’s Hospital in Boston developed a new approach that combines a minimally invasive, painless microneedle platform capable of absorbing cell-surrounding, biomarker-containing fluid from deeper layers of the skin with an ultra-sensitive, single-molecule detection method (Simoa) that detects often rare, yet relevant biomarkers with greater sensitivity than conventional methods.
Patients with melanoma, the most serious type of skin cancer in which pigment-producing cells proliferate uncontrollably, can benefit from existing immunotherapies, but by no means all of them do. More than half of patients do not respond to current immunotherapy treatments and many of those who do respond develop resistance to the drugs’ effects. In order to make the best treatment decisions, clinicians must be able to assess which patients respond well at the start of therapy and which ones continue or stop responding, in addition to generating more effective immunotherapies.
Because melanoma patients’ malignant skin lesions are easily accessible, using immunotherapies locally rather than systemically infusing them into the blood circulation could be an effective strategy to remove them. Monitoring the immune system’s response to therapy right at the tumor site, by sensitively and continuously assessing distinct biomarkers that signal the desired immune cell activation and inflammatory response, could also allow for better and more tailored patient care.
Rapid readout of the responses to melanoma therapy using microneedles may enable effective drug screening and patient stratification to maximize therapeutic benefits.
Natalie Artzi
Now, a research team at the Wyss Institute at Harvard University, MIT, and Brigham and Women’s Hospital in Boston has developed a new approach that integrates a minimally invasive, painless microneedle platform capable of absorbing the cell-surrounding, biomarker-containing fluid from deeper layers of the skin with an ultra-sensitive, single-molecule detection method (Simoa) that detects often rare, yet relevant biomarkers with higher sensitivity than conventional methods.
The researchers demonstrated the feasibility of their technique in a mouse melanoma model by treating malignant tumors with a new medication. The therapy kills tumor cells locally by combining non-invasive focused ultrasound (FUS), which generates heat at the tumor site, with the delivery of a previously developed nanoparticle-bound activator of an inflammation-inducing protein known as stimulator of interferon genes (STING). Advanced Functional Materials published the findings.
“Rapid readout of the responses to melanoma therapy using microneedles may enable effective drug screening and patient stratification to maximize therapeutic benefits,” said Wyss Associate Faculty member Natalie Artzi, Ph.D., who led the study. Artzi is also an Associate Professor of Medicine at Harvard Medical School (HMS) and a Principal Research Scientist at the Institute for Medical Engineering and Science at MIT.
Immunotherapy made local
Artzi and her colleagues initially created a locally applied immunotherapy for melanoma using some of their previously pioneered technologies and knowledge. They described a substantially more successful technique to activate the protein in immune cells in a recent paper, which drew on the known fact that activation of the inflammation-inducing STING protein adds to tumor cell destruction.
Natural STING activators are not sufficiently stable in the body and must be administered at high quantities, which can also cause side effects. The group’s answer was to deliver multiple copies of a synthetic STING agonist, known as a synthetic cyclic dinucleotide (CDN), via nanoparticles (NP) that easily cross the plasma membrane and release their cargo inside cells via an engineered enzymatic reaction. This CDN-NP therapeutic can be directly injected in or close to cancerous skin lesions to additionally increase the drug concentration in tumors.
“Here, we chose to boost the processing of antigens from dying tumor cells following focused ultrasound together with STING-agonist delivery in the tumor microenvironment to coordinate a broader immune response,” said first-author Daniel Dahis, Ph.D. Dahis, is a Research Scientist at the BioDevek startup, and performed his graduate work on the study with Artzi while being co-supervised by co-author Haim Azhari, Ph.D., a Professor at Technion Israel Institute of Technology and an expert in medical imaging.
The researchers first demonstrated that the focused ultrasound (FUS) treatment, which temporarily raises the temperature to 60°C in small areas, enhanced the effects of CDN-NP treatment in co-cultures of immune and cancer cells in a dish, as well as in melanoma tumors in mice treated with the combination. Importantly, all animals that received only FUS therapy perished 60 days later, whereas 75% of mice that received only CDN-NP therapy were still alive – the combination treatment allowed all animals in their group to survive.
Tapping deep into skin biomarkers
Artzi’s group had previously developed a novel type of minimally invasive microneedles made of hyaluronic acid that can be used to simultaneously deliver drugs and detect biomarkers to investigate if the survival benefits of the combination therapy are mirrored in the levels of biomarkers at the tumor site, which in the future could translate to the monitoring of responses in human patients treated with immunotherapy.
These devices reach into the lower (dermal) layers of the skin where the polymer encounters the fluid surrounding skin cells, the so-called interstitial skin fluid (ISF), and, like a sponge, takes up tiny amounts of it. “Merely a few microliters of ISF obtained with microneedles provide a wealth of biomarker information as normal skin cells, local immune cells, and cancer cells constantly secrete diverse signaling molecules and metabolites,” said Dahis. “After the microneedles are retrieved, their tips can be simply dissolved to release the captured molecules into a test tube for us to start the biomarker analysis.”
While FUS was definitely added to the immune response elicited by CDN-NP in tumors, numerous biomarkers of interest, including genes activated by the activated STING protein, were hardly detectable or not detectable at all using standard methods. Artzi’s team collaborated with Wyss Core Faculty member David Walt, Ph.D., who had previously created the Simoa technology, which has ultrasensitive biomarker detection capabilities, to overcome this bottleneck. Simoa essentially allows researchers to capture a biomarker protein of interest using a particular antibody molecule attached to a magnetic bead several times the size of the antibody itself.
The attached protein is then “sandwiched” with a second detector antibody in individual wells of a multi-well plate, each of which can only hold one magnetic bead. The detector antibody is tagged with an enzyme that causes a fluorescence signal to be generated in the well. Simoa therefore allows for the digital counting of individual biomarker proteins at the single-molecule level, significantly beyond the sensitivity of routinely employed detection methods.