Neutron star mergers are a goldmine for novel physics signals, with implications for understanding the true nature of dark matter, according to Washington University in St. Louis researchers.
On August 17, 2017, the Laser Interferometer Gravitational-wave Observatory (LIGO) in the United States and the Virgo detector in Italy observed gravitational waves from a collision of two neutron stars. For the first time, dozens of telescopes on the ground and in space saw this celestial event in light, in addition to hearing it in gravitational waves.
Bhupal Dev, a physicist at Arts & Sciences, used measurements from this neutron star merger, known in astronomical circles as GW170817, to establish new limitations on axions. Although these hypothetical particles have not been actually detected, they exist in numerous extensions of the standard model of physics.
Extreme astrophysical environments, like neutron star mergers, provide a new window of opportunity in our quest for dark sector particles like axions, which might hold the key to understanding the missing 85% of all the matter in the universe/
Bhupal Dev
Axions and axion-like particles are leading candidates to make up a portion or all of the universe’s “missing” stuff, or dark matter, which scientists have yet to explain. At the very least, these feebly interacting particles can act as a doorway, connecting the visible sector of the cosmos that humans are well-versed in to the unknown dark sector.
“We have good reason to suspect that new physics beyond the standard model might be lurking just around the corner,” said Dev, first author of the study in Physical Review Letters and a faculty fellow of the university’s McDonnell Center for the Space Sciences.
When two neutron stars merge, a hot, dense remnant is formed for a brief period of time. This remnant is an ideal breeding ground for exotic particle production, Dev said. “The remnant gets much hotter than the individual stars for about a second before settling down into a bigger neutron star or a black hole, depending on the initial masses,” he said.
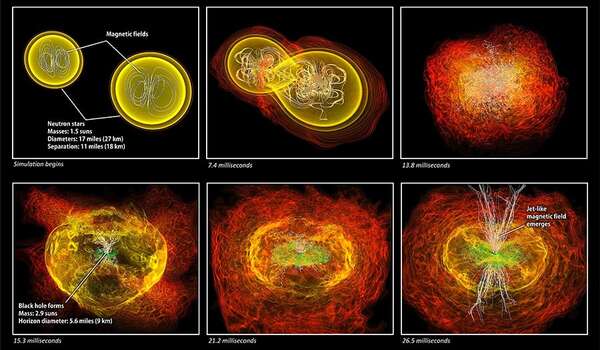
These new particles quietly escape the debris of the collision and, far away from their source, can decay into known particles, typically photons. Dev and his team — including WashU alum Steven Harris (now NP3M fellow at Indiana University), as well as Jean-Francois Fortin, Kuver Sinha and Yongchao Zhang — showed that these escaped particles give rise to unique electromagnetic signals that can be detected by gamma-ray telescopes, such as NASA’s Fermi-LAT.
The researchers examined the spectrum and temporal data from these electromagnetic signals and discovered that they could separate them from the known astrophysical background. Then, using Fermi-LAT data from GW170817, they derived new limitations on axion-photon coupling as a function of axion mass. These astrophysical restrictions complement those obtained from laboratory experiments such as ADMX, which investigate a distinct part of the axion parameter space.
In the future, scientists could use existing gamma-ray space telescopes, such as the Fermi-LAT, or proposed gamma-ray missions, such as the WashU-led Advanced Particle-astrophysics Telescope (APT), to collect additional data during neutron star collisions and improve their understanding of axion-like particles.
“Extreme astrophysical environments, like neutron star mergers, provide a new window of opportunity in our quest for dark sector particles like axions, which might hold the key to understanding the missing 85% of all the matter in the universe,” Dev said.