Gadolinium
Definition
Gadolinium is a silvery-white, malleable, ductile metallic element of the lanthanide series that has seven natural isotopes and 11 artificial isotopes. It is a Rare Earth Element (REE) with paramagnetic properties. On the Periodic Table its symbol is Gd and its atomic number is 64; atomic weight 157.25; melting point 1,312°C; boiling point approximately 3,000°C; specific gravity from 7.8 to 7.896; valence 3. Gadolinium is one of 15 metallic chemical elements known as the Lanthanide Series.

Gadolinium was discovered in 1880 by Charles Galissard de Marignac at Geneva. He had long suspected that the didymium reported by Carl Mosander was not a new element but a mixture. His suspicions were confirmed when Marc Delafontaine and Paul-Emile Lecoq de Boisbaudran at Paris reported that its spectral lines varied according to the source from which it came. Indeed, in 1879 they had already separated samarium from some didymium which had been extracted from the mineral samarskite, found in the Urals. In 1880, Marignac extracted yet another new rare earth from didymium, as did Paul-Émile Lecoq de Boisbaudran in 1886, and it was the latter who called it gadolinium.
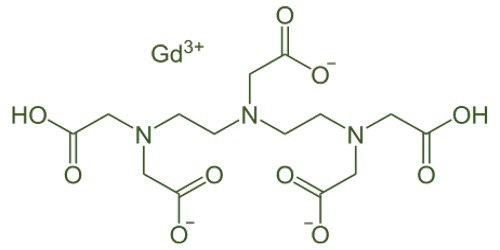
Gadolinium (Gd3+) has an ionic radius very similar to that of Calcium (Ca2+) which is why Gd3+ is so toxic in biological systems – Gd3+ can compete with Ca2+ in all biological systems that require Ca2+ for proper function and, in doing so, the trivalent Gd3+ ion binds with much higher affinity. Gadolinium is used to improve the heat and corrosion resistance of iron, chromium, and various alloys and in medicine as a contrast medium for magnetic resonance imaging and as a radioisotope in bone mineral analysis.

Production and Properties of Gadolinium
Gadolinium is a constituent in many minerals such as monazite and bastnasite, which are oxides. It is also prepared by reducing anhydrous gadolinium fluoride with calcium metal. The metal is too reactive to exist naturally. Ironically, as noted above, the mineral gadolinite actually contains only traces of Gd. The abundance in the earth crust is about 6. 2 mg/kg. The main mining areas are China, USA, Brazil, Sri Lanka, India and Australia with reserves expected to exceed one million tonnes. World production of pure gadolinium is about 400 tonnes per year. Gadolinium is a silvery-white malleable and ductile rare-earth metal. It crystallizes in hexagonal, close-packed 1- form at room temperature, but, when heated to temperatures above 1235 °C, it transforms into its 2- form, which has a body-centered cubic structure. Gadolinium is a strong reducing agent, which reduces oxides of several metals into their elements.
Gadolinium is a soft, bright, and a silvery metal having both the properties called malleable and ductile. It is the 66th element of the periodic table with its symbol being Gd.This is one of the rarely found metals on the earth. It is paramagnetic in nature at the room temperature and is ferromagnetic when cooled or at low temperatures of about 20oc. The Curie point of the metal gadolinium is about 17oc. Gadolinium has around 27 synthetic isotopes and about 13 number of naturally occurring isotopes.
Uses of Gadolinium
Gadolinium has useful properties in alloys. As little as 1% gadolinium can improve the workability of iron and chromium alloys, and their resistance to high temperatures and oxidation. It is also used in alloys for making magnets, electronic components and data storage disks. Its compounds are useful in magnetic resonance imaging (MRI), particularly in diagnosing cancerous tumours.

The alloys of gadolinium are utilized in the manufacture of magnetic and electronic gadgets such as video recorder, etc. These are highly used in the nuclear power reactors of the nuclear power plants as control rods. The isotope of gadolinium Gd is widely employed in curing tumors and neutron therapy
Gadolinium gallium garnet is used for the imitation of diamonds. The metal is also used for many high-temperature devices as it has good resistivity.
Reference: