Silver Chloride
Definition
Silver chloride is a white insoluble powder that darkens on exposure to light because of the production of metallic silver: used in making photographic emulsions and papers. Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by greyish or purplish coloration to some samples. AgCl occurs naturally as a mineral chlorargyrite.

Silver chloride is easily synthesized by combining aqueous solutions of silver nitrate and sodium chloride.
![]()
Structure of Silver Chloride
The chemical formula of silver chloride is AgCl, and its molar mass is 143.32 g/mol. Silver chloride is a simple ionic compound consisting of the silver cation (Ag+) and chloride anion (Cl-). In the solid state, AgCl adopts a crystalline structure similar to that of sodium chloride (NaCl), with each silver cation being surrounded by six chloride anions in an octahedral geometry.
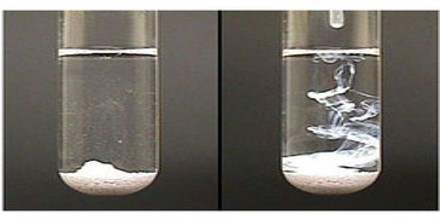
It is insoluble in water, alcohol and dilute acids but soluble in ammonia and concentrated acids. It is a photosensitive material (it undergoes chemical reactions in the presence of light), and upon illumination, it decomposes into silver metal and elemental chlorine. This reaction is characterized by the darkening of the AgCl sample, and makes AgCl an important chemical for photographic applications.
Uses and Effects of Silver Chloride

Many of the applications of AgCl are based on its light sensitive conversion to metallic silver, such as preparation of photographic films and photochromic lenses. It is used to make photographic paper since it reacts with photons to form latent image and via photoreduction. It is also used to create yellow, amber, and brown shades in stained glass manufacture, as an infra-red transmissive optical component as it can be hot-pressed into window and lens shapes. Silver chloride is not toxic at low concentrations and is used in medical and disinfecting applications. However, if swallowed or inhaled in high concentrations, it may cause irritation of mucous membranes, greyish discoloration of the internal tissues (argyria) and kidney damage. Skin or eye contact with silver chloride can cause greyish discoloration of the skin and tissues.