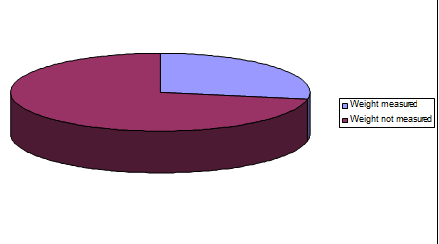INTRODUCTION
Dengue fever is caused by Dengue virus (a flavivirus). It has four serotypes producing similar clinical manifestation. These are Dengue fever, Dengue Hemorrhagic fever, Dengue Shock Syndrome. The vector of this virus is Aedes mosquito which lives in clear stagnant water. Due to absence of specific antiviral therapy and vaccines and the characteristic feature of vector, the therapeutic and preventive aspects are very demanding.
1.2 Rationale :
In 2000 for the first time Bangladesh experienced the toll of dengue and dengue haemorrhagic fever mostly in major cities with 5551 documented cases and 93 deaths. Subsequently Dengue fever became endemic in Bangladesh with more cases in each year. During the outbreak of dengue in Bangladesh, a national guideline for clinical management of Dengue Syndrome was adopted by customizing WHO/SEARO guidelines. Extensive training program was done for the working doctors of city hospitals where dengue patients were managed. But after implementing national guidelines death toll declined to reasonable level. Now Dengue has not a fear factor as it was in first 1-2 years of outbreak. It was made possible only by implementing national guideline for management of Dengue fever. So it is very important to see whether Dengue patients are identified and receives appropriate management according to national guideline.
2.AIMS AND OBJECTIVE:
To see whether Dengue patients are managed according to the national guideline in DMCH.
3. REVIEW OF LITERATURES
DENGUE FEVER (DF) AND DENGUE HAEMORRHAGIC FEVER (DHF)
3.1 INTRODUCTION
Dengue is a viral febrile illness, which has become a major international public health concern. It cases a spectrum of illness ranging from asymptomatic infection through relatively mild non-specific viral syndrome known as dengue fever (DF) to life threatening dengue haemorrhagic fever (DHF), dengue shock syndrome (DSS) and ultimately death.
The syndrome of dengue comprises (4) four entities undifferentiated fever (UF), dengue fever (DF), dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS)1,2. All entities initially present in a similar fashion with abrupt onset of fever, mylagia, arthralgia, rash and other non-specific features indistinguishable from one another.
Dengue haemorrhagic fever is not a complication of dengue fever rather than from the very onset starts as dengue haemorrhagic fever. The turning point between dengue fever and dengue haemorrhagic fever is the afebrile period, when dengue fever progresses to remit but dengue haemorrhagic fever progresses to its morbid manifestations. Dengue shock syndrome is a complication of dengue haemorrhagic fever. One cannot differentiate dengue Fever and dengue haemorrhagic fever at the very beginning of the illness. So it is preferable to encompass all the entities as dengue syndrome till the differentiating manifestations are surfaced3.
3.2 EPIDEMIOLOGY
The term “Dengue” has its origin in Zanzibar where the disease was called Dengue during 1870 epidemics 4. Epidemics of an illness compatible with dengue fever were first reported in the medical literature in 1779 and 1780 5. It is endemic in the Caribbean 6. Cases in the United States are mostly imported, but can be seen in the Gulf States as well as South East Asia 6. However, dengue hemorrhagic fever has reported as a new disease for the first time in Philippines in 1954 7,8, subsequently in Thailand in 1985, Myanmar in 1970 and finally in India in 1963 9,10. Dengue haemorrhagic fever is now an increasing public health problem in most of the Tropical countries of South-East Asia and Western Pacific regions 11,12. Dengue hemorrhagic fever and Dengue shock syndrome are now a leading cause of hospitalization among children in Asia 13,14. In 1964 there was an outbreak of dengue viral infection called “Dacca fever” in Bangladesh 15. Delhi witnessed epidemics of dengue fever in 1969. 1982 and in 1986 50-51. A major epidemic of dengue fever / dengue haemorrhagic fever / Dengue shock syndrome occurred during September to November in 1986 and all age groups were affected 16-18. in all aspects the geographically contagious countries surrounding Bangladesh with which we have good and fast communications and relations have dengue with endemic propensity and regular epidemics as a great public health problem.
In 1999 an outbreak of dengue fever with few unconfirmed cases of dengue haemorrhagic fever was reported in and around Dhaka city in Bangladesh as suggested by positive serological evidences. During July to December 2000. Bangladesh has witnessed a large-scale outbreak of this dreaded disease mainly in urban areas. According to leading researchers, coming years may presage even larger scale outbreaks.
PREVALANCE
The global prevalence of dengue has grown dramatically in recent decades.
It is endemic in more then 100 countries in Asia, Americas, Africa, South-East Asia and Western pacific.
Some 2500 million people are at risk of infection.
WHO estimates 50-100 million cases of dengue infection occur annually.
Over half of the world’s population live in areas at risk of infection and these ,are popular tourist destinations also.
3.3 THE VIRUS
Dengue virus belongs to the family Flavivirdat and Genus Flavivirus. The Flavivirus virion is a spherical structure approximately 50 nm in diameter consisting of a single strand RNA genome located inside a nucleocapsid which is in turn surrounded by a protein and lipid envelope.
The virus has four flavors called serotype, which are creatively named as DEN-1, DEN-2, DEN-3, DEN-4, All the viral proteins are coded in a single, long open radiating frame about 10.5 kb in length. All the serotypes can cause dengue haemorrhagic fever/dengue shock syndrome. Severe disease is common with DEN-2 and DEN-3 and have a tendency to be neurovirulent.
3.4 IMMUNITY
Infection with a particular dengue serotype offers long lasting homotypic immunity. Heterotypic immunity lasts for a brief period of months after which patients are susceptible to infection with another serotype 22. Therefore infection with one serotype does not offer protection against other serotype. In-fect a second dengue infection leads to an even worse infection and manifests as dengue haemorrhagic fever or dengue shock syndrome, which can be fatal23
3.5 THE VECTOR:
The main vector of dengue is female Adedes aegypti flourishing in mankind’s urban to suburban environments. Another mosquito Aedes albopictus is a less important urban vector, and plays an important role to spread the disease in Asian region. Aedes polynesinensis is another one considered 6,The main vector Aedes aegypti, a domestic mosquito is thought to have been introduced to Asia Africa 24,25. Aedes albopictus, a mosquito present in the vegetation particularly in forested areas 26. • ,
The tropical zones of the world between 35°S latitude and area not over 1000 feet above sea level are the usual habitat. These areas are marked by monsoon rains. The breeding is highest during pre and post monsoon periods27. Aedes aegypti breed hi clean stagnant water.
The number of automobiles has increased over the last few years in a phenomenal proposition leading to increased number of waster tyres that are providing a good site for clean stagnant water for the Aedes aegypti to breed and multiply by catching monsoon rains . A factor complicating eradication of the vector is that Aedes aegypti eggs can withstand long periods of desiccations, sometimes more than a year 29.
While the eggs, larval and pupal survival is independent of a wide range of temperature the immature forms of aedes aegypti do suffer significant mortality at temperature below 5°c and above 42°c30-32
3.6 THE HOST
Man is the principal mammalian host. The mosquito is another important reservior host for dengue virus as well. All four virus serotypes are maintained in a mosquito-human mosquito cycle in most urban centers of the tropics where no other reservior hosts are present or needed. It is this pool of viruses that plays an important role in the current global resurgence of epidemic dengue in recent years primarily by airplane travelers.
3.7 PATERN OF DENGUE VIRUS TRANSMISSION
Dengue virus is not contagious and person-to-person transmission does not occur. Transmission to a human needs an infective mosquito to bite an individual for blood meal. Multiple feedings or biting by an infective mosquito may transmit to multiple persons in the same household and all having onset of illness within a few days 33,34.
Mosquitoes become infected when they take blood meal from a viraemic person. Viraemia is present for about 24 hours prior to onset and for an average of 5 days after onset of illness usually coinciding with the period of fever. Once infected the mosquito can transmit the virus throughout its life. Infected female mosquito can transmit the virus to its next generation by transovarian route 15.
The biting seems to occur throughout the day but marked by two distinct peaks, morning peak at 0800 hrs to 1300 hrs and mid afternoon peak between 1500 hrs to 1700hrs. Vertical transmission of dengue virus has been recorded in a small number of cases leading to neonatal dengue fever or even dengue shock syndrome transmission from a needle stick injury has been reported One case of nosocomial
3.8 INCUBATION PERIOD
> The average incubation period is 4-6 days.
> It may be as short as 3 days and as long as 14 days.
3.9 EXTRINSIC INCUBATION PERIOD
This is the time between the entry of the virus into the mosquito and becoming infective to other persons. During this time the viruses grow within the mid-gut and infects a number of tissues in the mosquito including the salivary gland. After this period it can transmit dengue viruses by taking a blood meal or by simply probing the skin of a susceptible person33,34.
3.10 PATHOGENESIS
PRIMARY INFECTION
Once the virus enters into the human body it is taken up by mononuclear phagocytes where the viruses replicate. Infection of megakaryocytes causes abnormal megakaryocytopoiesis and there is also marrow suppression due either to direct infection or to release of cytokines down regulating haematopoiesis or both 34. Viral particles or antigens have been detected in monocytes of kidney, skin tissues, liver, spleen, thymus, lungs, lymph nodes and heart. There is some evidence that dengue virus can infect CNS 39,40. Pathogenesis of classical Dengue fever is like that of other viral illness.
ANTIOBODY RESPONSE IN PRIMARY INFECTION
IgM antibody is produced due to first infection after a period of 6-7 days. Then after a certain period it becomes negative (3 months). IgG antibody response starts after the response of IgM and persists for life, gives protection against subsequent infection by the same serotype.
SECONDARY INFECTION
Preformed IgG is the mainstay in the pathogenesis of dengue haemorrhagic fever/dengue shock syndrome. Subsequent infection by other strains leads to immune enhancement due to non-neutralizing antibody level. The most widely accepted hypothesis for the pathogenesis of dengue haemorrhagic fever in secondary infection is the immune enhancement44,45. Antibody dependant enhancement of dengue viruses is a process in which the infecting virus is complexed with non-neutralizing antibody, thus enhancing phagocytosis by mononuclear cells. Virus replication induces infected monocytes to release vasoactive mediators and activation of the complement system which results in the vascular permeability and haemorrhagic manifestations that characterize dengue haemorrhagic fever and dengue shock syndrome46-50.
Another hypothesis proposes the involvement of delayed hypersensitivity reaction in the pathogenesis of dengue haemorrhagic fever. It is possible that the dengue virus specific T-cells contribute to the pathogenesis of dengue haemorrhagic fever / dengue shock syndrome by producing IFN y and lysis of dengue virus infected cells during secondary infections 51.
The presence of circulating antibody acquired actively by prior infection or passively by heterotypic maternal dengue IgG antibody in infants with primary infection -is the most frequently reported cause of altered response to dengue45-46
Epidemiologic and laboratory evidence suggests that the virus strains and perhaps serotype may also be important as a risk factor for dengue haemorrhagic fever Dengue haemorrhagic fever/dengue shock syndrome with fatalities has been documented in adults and children with primary7 infection 52,53. Ironically undernourished infants seem to have a lower risk of dengue haemorrhagic fever then infants with good nutritional status94. Dengue shock syndrome is rarely seen in critically malnourished children55. However- cytokines and complement activation lead to the following path physiologic abnormalities.
« Increased vascular permeability- Leads to pleural effusion, Ascites, Haemoconcentration, hypovolaemia. Shock, Renal failure and Acidosis.
• Abnormal haemostasis- Occurs by combination of coagulation defects. Thrombocytopenia. platelet dysfunction, Vasculopathy which lead to DIG. Haemorrhage, shock, HUS, renal failure and Acidosis.
• Thrombocytopenia- This is due to megakaryocytic dysfunction, destruction of mature platelets and consumpation coagulopathy leading to haemorrhage.
4. NATIONAL GUIDELINE FOR CLINICAL MANAGEMENT OF DENGUE SYNDROME 3
4.1 Manifestations of Dengue Infection
There are four sero types of dengue virus, Den-1, Den-2, Den-3 and Den-4. All four types following infection produce similar manifestations, which may be asymptomatic, undifferentiated fever, dengue fever (DF), dengue hemorrhagic fever (DHF) with plasma leakage, that may lead to hypovolumic shock, dengue shock syndrome (DSS). In initial period manifestations are similar and deteriorating from one state to another.
Manifestations of dengue virus infection
A symptomatic
Undifferentiated Fever
Without hemorrhage
Symptomatic Dengue Fever
With Hemorrhage
No shock
Dengue Hemorrhagic Fever
Shock
Dengue Syndrome
The symptomatic manifestations for all practical purpose are overlapping in nature, and not differentiable at the beginning, some time appears progressing from one category to another. So they are grouped into ‘Dengue Syndrome’. Dengue syndrome will encompass the following:
1. Dengue Fever (DF)
2. Dengue Hemorrhagic Fever (DHF)
3. Dengue Shock Syndrome (DSS)
Purposes of Case definitions
There are two purposes for case definition, which are:
1. For clinical case management in hospital setups and in outpatient practice.
2. For reporting of the cases to designated appropriate health authority.
4.2 Case Definitions-for Clinical Management
Dengue Fever
Dengue fever is an acute febrile illness of 2-7 days duration sometimes with two peaks having the following manifestations:
1. Sudden onset continuous fever
And
2. Two or more of the following features:
a. Severe headache
b. Retro-orbital pain
c. Severe myalgia / arthralgia / back pain
d. Hemorrhagic manifestations
e. Nausea/vomiting/abdominal pain
f. Leucopenia
And
3. High index of suspicion based on Period, Population & Place
And
4. Absence of convincing evidence of any other febrile illness
Dengue Hemorrhagic Fever
Dengue Hemorrhagic Fever is a probable manifestation of dengue syndrome with hemorrhagic manifestations having the following features:
1. Features of dengue fever at initial stage
And
2. Hemorrhagic manifestations evidenced through one or more of the following:
a. Positive tourniquet test
b. Petechiae / ecchymosis / purpura
c. Mucosal bleeding: Epistaxis, gum bleeding
d. Bleeding from injection or other site
e. Hematemesis, melena, hematuria, PV bleeding
f. Thrombocytopenia with platelets 100,000 /mm’ or less
And
3. Any evidence of plasma leakage due to increased capillary permeability manifested by one or more of the following:
a. A 20% rise in hematocrit for age or sex
b. A 20% drop in hematocrit following treatment with fluids as compared to base line
c. Pleural effusion / ascitis / hypoproteinemia
The cut-off point between Dengue Fever and Dengue Hemorrhagic Fever is the evidence of plasma leakage, which will not be present in the former but invariably in the later. This is important in differentiating DF with hemorrhage from DHE
Tourniquet Test
This is a very important clinical test for detecting covert hemorrhage. It is performed by inflating a blood pressure cuff to a point midway between the systolic and diastolic pressures for five minutes. A test is considered positive when 10 or more petechiae per 2.5 –cm2 are observed. In DHF, the test usually gives a definitive positive result ie > 10 petechiae. The test may be negative or mildly positive during the phase of profound shock.
Typical rash of dengue haemorhhagic fever
Dengue Shock Syndrome
Dengue Shock Syndrome is a presentation of Dengue Syndrome when a case of DHF manifests circulatory failure with one or more of the following features:
1. Hypotension for age
2. Cold clammy skin, restlessness, rapid weak pulse
3. Narrow pulse pressure (<_ 20 mm of Hg) .
4. Profound shock
Case Definitions for Reporting
Dengue is a notifiable disease. For the purpose of the notification to appropriate health authority the case definitions are as follows:
1. Suspected: Clinically diagnosed as per ‘Clinical Case definition’.
2. Probable: When in addition to clinical diagnosis any serological or rapid test is found to be positive.
3. Confirmed: When the case is confirmed by virus isolation.
When reporting the ‘reporting case definition’ term will be added as a prefix to the ‘clinical case definition’ term of dengue syndrome categorized as per national guidelines.
Example
A case is found to be Dengue Fever as per clinical case definition. For reporting purpose this will be labeled as ‘Suspected Dengue Fever’. If any serological test is found positive this case will be ‘Probable Dengue Fever’ and so on.
4.3 Severity Grading of Dengue Syndrome
Syndromes Grades Clinical features Laboratory features
DF Features of DF as per case definition • Leucopenia
• ± Thrombocytopenia
• No change in hematocrit
DHF I Features / History of features of DF • Thrombocytopenia
+ • 100,000 /mm3
Positive Tourniquet Test • Hematocrit rise 20%
DHF II Features / History of features of DF • Thrombocytopenia
+ • < 100,000/mm
Spontaneous bleeding • Hematocrit rise 20%
DHF (DSS) III Features / History of features of DF • Thrombocytopenia
+ • < 100,000/mm3
Features of circulatory failure • Hematocrit rise 20%
DHF (DSS) IV Features’/ History of features of DF • Thrombocytopenia
+ • < 100,000 /mm3
Profound shock • Hematocrit rise 20%
DHF Grades III & IV are also called Dengue Shock Syndrome (DSS).
• At the initial phase one cannot differentiate DF and DHF.
• The course is a continuum passing from one grade to another.
• The transition period is at the afebrile phase
• If appropriate treatment is not instituted in proper time there is a risk of death in DHF-II, DHF-III and DHF-IV.Disease Course of Dengue Syndrome
The disease course in dengue syndrome has the following characteristics:
A. Phases: There are two phases from the beginning.
a. Febrile phase: Which lasts from 2-7 days duration. Various categories of presentation cannot be differentiated at this stage.
b. Afebrile/ Critical phase: This phase follows the febrile phase and lasts for 2-3 days. The patient is afebrile. In DF cases this phase may be called afebrile phase and usually marks the beginning of convalescence. But in DHF cases at this stage all critical features begin and is called the critical phase.
B. Progression: The natural course of progression of dengue syndrome is a continuum, uphill, stationary or downhill mostly not distinguishable at the initial stage. It has to be remembered that DHF per see begins as DHF and not converting from or a complication of DF, but indistinguishable from DF at the beginning.
Dengue Syndroime: DF/DHF
Febrile : Phase.- 2-7 days
Dengue Syndrome: DF/DHF DHF/DSS
Afebrile/Critical Phase: 2-3 days * * Death
DHF-I DHF-II DHF-III DHF-IV __
Convalescent Phase
* If appropriate treatment is not provided then there is high risk of death.
4.4 Lab investigations: For diagnosis & Prognosis
The useful lab investigations are as follows:
I. Complete Blood Count (CBC) including Total Leucocyte Count, Total Platelet Count and Hernatocrit
2. Chest X-Ray right lateral decubitus view or Ultrasonography (for pleural effusion or ascitis)
3. Other routine tests as indicated eg MP for excluding malaria in malaria endemic zone
4. Other tests as and when necessary eg Serum Albumin, Liver function tests, Serum electrolytes
Time and frequency of doing investigations:
Usually before 3 days no change in the lab tests is expected in febrile phase. So no tests should be done before 3 days if not otherwise indicated eg unusual hemorrhage. But once clinically suspected leucocyte and platelet counts plus hematocrit level should be done at least once per day.
Tests for objective evidence of dengue infection is not helpful for guiding the management. Moreover doing this at initial period will usually give negative result there by provides a false sense of security to the patients and doctors.
Leucocyte count has a very important prognostic guide in early phase of dengue infection. Leucopenia
Serial Leucocyte Count, Hematocrit level and Platelet Count are very important for prognostic purpose.
Treatment of Dengue Syndrome
Febrile Phase: Therapy
DF and DHF are not distinguishable in febrile phase and treatment is essentially same. The modality of treatment is symptomatic and supportive. These are:
• Rest
• Antipyretic therapy for fever above 39° C.
a. Sponging: With tepid water at room temperature.
b. Paracetamol (not more than 4 times in 24 hours) according to age
Age | Dose (500 mg tablet) | Mg/Dose |
< 1 Year | 1/8 tablet | 62.5 |
1- 4 Years | 1/4 tablet | 62.5-125 |
³ 5 Years | 1/2 tablet | 250 |
• Do not give Aspirin or any other NSAID. These drugs may cause gastritis and or bleeding. In children, Reye’s syndrome may be a serious complication.
• Do not give antibiotics as these do not help.
• Oral Rehydration Therapy (ORT) with Oral Rehydration Salt (ORS) or its equivalent is recommended for patients with moderate dehydration caused by vomiting and high temperature .
• Food should be given according to appetite. But fresh fruit juice should be given frequently. Avoid commercially available fruit juices because these contain preservatives.
• In case of infant and children if there is febrile convulsion and or history of so appropriate standard measures should be taken.
Febrile Phase: Monitoring & Observation
All dengue patients must be carefully observed for complications for at least 2 days after recovery from fever. This is because life-threatening conditions often occur during this phase. Patients and households should be informed that severe abdominal pain, passage of black stools, bleeding into the skin or from the nose or gums, sweating, and cold skin are dangerous signs. If any of these signs is noticed, the patient should be taken into the hospital. The patient who does not have any evidence of complications and who has been afebrile for 2-3 days does not need further observation.
Patient and household members should be informed by the doctor that abdominal pain, passage of black stools, any bleeding including undue PV bleeding, sweating and cold skin are dangerous signs, and if any sign(s) is noticed, the patient should be taken to hospital immediately.
Afebrile Phase: Dengue Fever
Constitutional symptoms in patients with DF after the fall of fever are similar during the febrile phase. Most patients will recover without complication. The following manifestations may present:
• Improvement in general condition
• Platelet/Hematocrit normal
• Appetite rapidly regained
Management is more or less same, ie continue bed rest, check platelet and hematocrit; fruit juices, oral fluid and electrolytes therapy.
Convalescent Phase: Dengue Fever
The duration of convalescence phase is 7-10 days after the afebrile phase. During this phase further improvement in general condition and return of appetite occur. Bradycardia and confluent petechial rash with white center and or itching may persist. Weakness may remain up to another week or two. No special advice is necessary. No restriction is also needed. Normal diet and effort for adjusting to normal life style and work are what is necessary.
Critical Phase: DHF
During the afebrile phase usually the features of DHF evolve, which are various bleeding manifestations, signs of circulatory failure and, progressive thrombocytopenia and plasma leakage as manifested by rise in hematocrit. Depending on the grading of severity the management should be instituted immediately to avoid fatality. Therefore this period is very crucial. Moreover the only difference between DF and DHF Grade I is the presence of thrombocytopenia and rise in hematocrit 20%.
Indication far hospitalization in Dengue Syndrome
• DF cases with any one or all of the following conditions: monitoring and observation cannot be ensured, with high risk associated illness, nutrition and, therapy cannot be maintained, and in special situations. Otherwise there is no indication for hospitalization for DF cases.
• DHF Grades II, III & IV.
• DHF Grade I where nutrition and oral fluid electrolytes therapy, monitoring and observation cannot be ensured, and or presence of concomitant illness or in special situations eg Diabetes, IHD, Pregnancy, etc.
Main objectives of therapy in DHF
• Maintenance of fluid and electrolytes
• Maintenance of blood osmolarity in face of plasma leakage
• Maintenance of circulatory volume and hemodynamic status
• Maintenance of nutrition
• Prevention of complications
Critical Phase: DHF I&-II Therapy
During the afebrile phase of DHF Grades I & II, the patient has the same symptoms as during the febrile phase. The clinical signs plus thrombocytopenia and hemoconcentration or rise in hematocrit are sufficient to establish a clinical diagnosis of DHF. During this phase, the patient should be observed for at least 2-3 days after the fall in temperature, for rashes on the skin, bleeding from nose or gums, blue spots on the skin or tarry stools. If any of these signs are observed, the patients should be brought to the hospital without delay.
DHF Grades I & II Therapy Chart | ||
1.1.1 Critical Phase | Manifestations | Management |
| Duration 2-3 days | – Features of DF – Positive Tourniquet Test – Spontaneous bleeding – Thrombocytopenia <100,000 /mm[1] – Hematocrit rise ³ 209,
| – OPD or Hospital – ORS – Check Platelet / Hematocrit, if Hematocrit ³ 20% – Initiate IV therapy 5% DNS 6 ml/Kg/hr for 6 hours – Check hematocrit/ vital signs / urine output after 3 hours, and in case of improvement[2] – Reduce IV therapy to 3 ml/kg/hour for 3 hours – In case of further improvement, continue IV therapy at 3 ml/kg/hour for 6-12 hours and then discontinue IV therapy – In case of no improvement, increase IV therapy to 10 ml/k,/hour for 1 hour. In case of improvement now, reduce the volume of IV from 10 ml/kg/hour to 6 ml/kg/hour and further to 3 ml/k,/hour accordingly. – Generally, DHF Grades 1& II do not live complications |
| Convalescence Phase | Manifestations | Management |
| Duration 2-3 days | – Further improvement in general condition and return of appetite – Bradycardia – Confluent petechial rash with white center/ itchina – Asthenia and depression even persisting for few weeks, common in adults | – Normal diet – No need for any medication |
I is the presence of thrombocytopenia and rise in hematocrit (>20%). Patients with DHF Grade I do not usually require intravenous fluid therapy and ORT is sufficient. Intravenous fluid therapy may need to be administered only when the patient is vomiting persistently or severely, or refusing to accept oral fluids. Patients with DHF Grade I who live far away from the hospital or those who are not likely to be able to follow the medical advice should be kept in the hospital for observation.
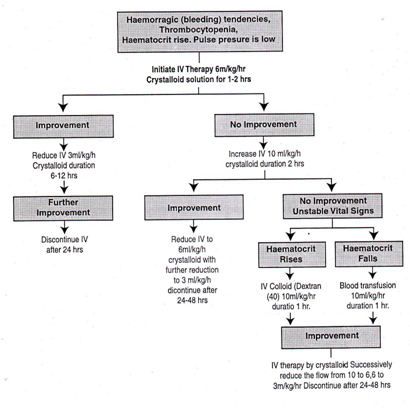
During the afebrile phase of DHF Grade II, the complications usually seen, in addition to those observed during DHF Grade I phase, are abdominal pain, black tarry stools, epistaxis, bleeding from the gum, and continued bleeding from the injection sites. Immediately after hospitalization, hematocrit and platelet count must be carried out to assess the condition of the patient. A reduction in platelet count to <_ 100,000/mm or less than 1-2 platelets/oil field (average of 10 oil field counts) usually precedes a rise in hematocrit. A rise in hematocrit of 20% or more (eg increase from 35% to 45%) reflects a significant plasma loss and indicates the need for intravenous fluid therapy. Early volume replacement of lost plasma with crystalloids solution (eg isotonic saline solution) can reduce the severity of the disease and prevent shock. Intravenous fluid therapy before leakage is not recommended. But in DHF Grade II depending on the condition IV therapy may given for 12-24 hours. Medical personnel should monitor patients on hourly basis. Based on periodic hematocrit/ platelet count determinations and vital signs, the treatment should be reviewed and revised. DHF Grades I & II: Volume Replacement Flow Chart Improvement: Hematocrit falls. pulse rate and blood pressure stable, urine output rises No improvement: Hematocrit/pulse rate rises, pulse pressure falls below 20 mm Hg, urine output falls Unstable vital signs: Signs of shock, urine output falls When hematocrit cannot be done or is not available the following clinical tips may help: • If the patient has/ had deep/massive bleeding form gut or other sites the possibility is that the patient may have lower hematocrit because of blood loss. • If the patient has/had scn facehnild bleeding the possibility is that the patient may have higher hematocrit.. • Sudden unexplained deterioration of henzodynarnic status and or refractory to adequate fluid therapy the possibility is more of blood loss and hence low hematocrit level. Critical Phase; DHF III & IV Therapy Common signs of complications observed during the afebrile phase of DHF Grade III include circulatory failure manifested by rapid and weak pulse, narrowing of the pulse pressure and hypotension, characterized by high diastolic pressure relative to systolic pressure (eg 90/80 mm of Hg) and the presence of cold clammy skin and restlessness. These complications occur because of thrombocytopenia, abnormal hernostasis and plasma leakage, or also from substantial blood loss. Immediately after hospitalization, the hematocrit, platelet count and vital signs should be examined to assess condition of the patient, and intravenous fluid therapy should be started. The patient requires regular and sustained monitoring. If the patient has already received about 1000 ml of intravenous fluids and the vital signs are still not stable, hematocrit should be repeated and: (a) if the hematocrit is increasing intravenous fluid should be changed to colloidal solution preferably Dextran, or (b) if hematocrit is decreasing, fresh whole blood transfusion 10 ml/kg/dose should be given. During the afebrile phase of DHF Grade IV vital signs are unstable. The patient, in the early stage of shock, has acute abdominal pain, restlessness, cold and clammy skin, rapid and weak pulse. The patient should be administered intravenous fluid therapy immediately. In case of continued or profound shock when pulse and blood pressure are undetectable, the patient should be given colloidal fluid following the initial fluid bolus. However, in the case of persistent shock when, after initial fluid replacement and resuscitation with plasma expanders, the hernatocrit continues to decline, internal bleeding should be suspected. It may be difficult to recognize and estimate the degree of internal blood loss in the presence of hemoconcentration. It is thus recommended to give fresh whole blood in small volumes of 10 ml/kg body weight at one time. Blood grouping and matching should be done for all patients in shock as a routine precaution. Oxygen should be given to all patients in shock.
DHF Grades III & IV Therapy Chart | ||
| Critical Phase | Manifestations | 1.1.1.1 Management |
| Duration two days after febrile stage | In addition to the manifestations of DHF Grade II – Circulatory failure manifested by rapid and weak pulse, narrowing of pulse pressure (20 nunHa or less) or hypotension with the presence of cold clammy skin and restlessness – Capillary refill time[1] more than two seconds
Profound shock with undetectable pulse and blood pressure | – Mandatory in Hospital Check hematocritlplatelet – Initiate IV therapy 501o DNS 10 ml/kg/hour – Check hematocrit, vital signs, urine output every hours – If patient improves, IV fluids should be reduced every hour from 10 to 6, and from 6 to 3 ml/ kg/hour which can be maintained up to 24 to 48 hours. – If patient has already received one hour treatment of 20 ml/kg/hour of IV fluids and vital signs are not stable, check hematocrit again and – If hematocrit is increasing change IV fluid to colloidal solution preferably Dextran or Plasma at 10 ml/kg/hour – If hematocrit is decreasing from the initial value, give fresh whole blood transfusion, 10 ml/kg/hour and continue fluid therapy at 10 ml/kg/hour and reducing it stepwise bring down to 3 ml/kg/hour and maintain it up to 24-48 how – Initiate IV therapy 5% DNS 20 makes a bolus one or two times – Oxygen therapy should be given to all patients[2] – In case of continued shock, colloidal fluids (Dcxtran or Plasma) should be given at 10-20 ml/kg/how -If shock still persists and hematocrit level continues declaiming fresh whole blood 10 ml/kg as a bolus – Vital signs should be monitored every 30-60 minutes – In case of severe bleeding, give fresh whole blood 20 ml/kg as a bolus – Give platelet rich plasma transfusion exceptionally when platelet counts are below 5,000-10,000/tnm’ – After blood transfusion, continue fluid therapy at 10 ml/kg/hour and reduce it stepwise to bring it down to 3 ml/kg/hour and maintain it for 24-48 hours. |
DHF Grades III & IV Therapy Chart | ||
| Critical Phase | Manifestations | 1.1.1.1 Management |
| Duration 2-3 days after recovery from critical phase | – 6-12 hours after critical/shock stage, some symptoms of respiratory distress (pleural effusion or ascitis) – 2-3 days after critical stage, strong pulse, normal blood pressure – Improved general condition/ return of appetite – Good urine output – Stable hematocrit – Platelet count >50,000/mm’ – Patient could be discharged from hospital 2-3 days after critical stage – Bradycardia/arrhythmia – Asthenia and depression | – Rest for 1-2 days– Normal diet– No need for medication
|
DHF Grades III & IV : Volume Replacement Flow Chart
Special Clinical Situations
DF and DHF may develop in a patient with some other clinical situations besides leading to others. Some common situations are as follows:
l. Pregnancy and labor
2. Emergency surgical condition eg Acute appendicitis
3. Associated medical conditions eg Diabetes mellitus, Myocardial infarction
4. Conditions where patients are on maintenance therapies which are contraindicated for DF/DHF eg Nephrotic syndrome case on high dose of steroid
5. Patient is on some procedure which may be complicated by DF/DHF eg Maintenance hemodialysis where heparin is used to increase clotting time.
6. Hypersensitivity or anaphylaxis due to fluid therapy in DF/DHF
General rule
In such situation the general rule of risk versus gain should be followed. Which are:
1 If avoidable, concomitant therapy which is contraindicated in dengue and or may create complication should be deferred or be avoided eg maintenance hemodialysis
2. In case of pregnancy and labor all patients should be hospitalized and carefully monitored. If possible labor should be avoided during critical phase. Other wise if not possible then all possible precaution should be taken to perform the obstetric procedure.
3. In case of emergency surgical condition conservative management should be adopted and surgery should be avoided if possible till the patient attains convalescence phase. But if the acute surgical condition is more risky than the DF/DHF then after taking adequate precaution life saving procedure may have to be adopted.
4. Concomitant medical condition if demanding enough to save life than the management should be continued.
5. If a patient is on maintenance therapy with high dose steroid then this should be continued.
6. In all such complicated situations a team approach comprising relevant specialists should be adopted.
7. Hypersensitivity and anaphylaxis during fluid therapy should be dealt with as per standard procedure.
In any complicated situation frequent consultations with other colleagues and multi disciplinary team approach are warranted.
Fluids Required for Intravenous Therapy
Fluids Recommended
Crystalloids
1. 5% dextrose in isotonic normal saline solution (5%DNS)
2. 5% dextrose in half strength normal saline solution (5%D/l/2/NS)
3. 5% dextrose in lactated Ringer’s solution (5%DRL)
4. 5% dextrose in acetated Ringer’s solution (5%DRA)
Colloids
1. Dextran 40
2. Hemacel
3. Plasma
Precautions
In order to ensure adequate fluid replacement and avoid over-fluid infusion, the rate of intravenous fluid should be adjusted through out the 24 to 48 hour period of plasma leakage by periodic hematocrit determinations and frequent assessment of vital signs. The volume of fluid replacement should be just sufficient to maintain effective circulation during the period of plasma leakage. Excessive fluid replacement and continuation for a longer period after cessation of leakage will cause respiratory distress from massive pleural effusion, ascitis, and pulmonary congestion or edema. This can be dangerous.
Fluid Requirement Calculation: Ready Reference
The required regimen of fluid should be calculated on the basis of body weight and charted on a 1- 3 hourly basis or even more frequently in the case of shock. The regimen of flow of fluid and the time of infusion are dependent on the severity of DHF. The schedule given here is recommended as a guideline. It is calculated for moderate dehydration of about 6% deficit plus maintenance.
ml/lb Body weight/day | Weight on admission | ml/k Body weight/day | |
lbs | kgs | ||
100 | <15 | <7 | 220 |
75 | 16-25 | 7-11 | 165 |
60 | 25-40 | 12-18 | 130 |
40 | >40 | >18 | 90 |
In older children who weigh more than 40 kgs, the volume needed for 24 hours should be calculated as twice that required for maintenance (using Holliday and Segar formula). The maintenance fluid should be calculated as follows:
| Body weight (kgs) | Maintenance volume (ml) Administered over 24 hours |
<10 | 100/kg |
10-20 | 1000-50 for each kg in excess of 10 |
>20 | 1500+20 for each kg in excess of 20 |
Example: For a child weighing 40 k-s the maintenance is: 1500+(20×20)=1900 ml. This means that the child requires 3800 ml IV fluid during 24 hours.
Fluid Regimens in DHF: Ready Reference
For intravenous fluid therapy of patients with DHF, four regimens of flow of fluid are suggested: 3 ml/kg/hour, 6 ml/kg/hour, 10 ml/kg/hour and, 20 ml/kg/hour.
Body weight (in kg) | Volume of fluid to be given in 24 hours | Rate of fluid (ml/hour) Regimens (R) | |||
R-1 3ml/kg/hour | R-2 6ml/kg/hour | R-3 10mI/kg/hour | R-4 20m1/kg/hour | ||
10 | 1500 | 30 | 30 | 100 | 200 |
15 | 2000 | 45 | 60 | 150 | 300 |
20 | 2500 | 60 | 90 | 200 | 400 |
25 | 2800 | 75 | 120 | 250 | 500 |
30 | 3200 | 90 | 150 | 300 | 600 |
35 | 3500 | 105 | 180 | 350 | 700 |
40 | 3800 | 120 | 210 | 400 | 800 |
45 | 4000 | 135 | 240 | 450 | 900 |
50 | 4200 | 150 | 270 | 500 | 1000 |
55 | 4400 | 165 | 300 | 550 | 1100 |
60 | 4600 | 180 | 360 | 600 | 1200 |
• The fluid mentioned is approximation.
• Normally change should not be drastic. Do not jump from R-1 to R-4 since this can overload the patient with fluids. Similarly, reduce the volume of fluid from R-4 to R-3, R-3 to R-2, and from R-2 to R-1 in a stepwise manner.
• REMEMBER that ONE ml is equal to 15 drops in standard MACRO infusion set. In MICRO system (Micro burette infusion set) 60 drops are equal to 1 ml.
• It is advised to procure only a bag of 500 ml initially, and order more as and when required. The decision about the speed of fluid should be reviewed every 1-3 hour. The frequency of monitoring should be determined on the basis of the condition of the patient. The higher the flow rate the more frequent should be the monitoring.
Some Important Instructions
Check list
• Cases of DHF should be observed every hour.
• Serial platelet and hematocrit determinations for drop in platelets and rise in hematocrit are essential for early diagnosis of DHF.
• Timely intravenous therapy – isotonic crystalloid solution – can prevent shock and or lessen the severity.
• If patient’s condition becomes worse despite giving 20 ml/kg/hour, replace crystalloid solution with colloid solution such as Dextran or plasma. As soon as improvement occurs replace with crystalloid.
• If improvement occurs, reduce the speed from 20 ml to 10 ml, then 6 ml, and finally to 3 ml/kg.
• If hematocrit falls, give blood transfusion 10 ml/kg and then give crystalloid IV fluids at the rate of 10 ml/kg/how-.
• In case of severe bleeding, give blood transfusion about 20 ml/kg for two hours. Then give crystalloid at 10 ml/kg/hour for a short time (30-60 minutes) and later reduce the speed.
• In case of shock, give oxygen.
• For correction of acidosis (sign: deep breathing), use sodium bicarbonate .
• Check for any concomitant other medical or surgical condition and or any maintenance therapy.
Don’ts
• Do not give aspirin or NSAID for the treatment of fever.
• Avoid giving intravenous therapy before there is evidence of hemorrhage or bleeding.
• Avoid giving blood transfusion unless indicated, reduction in hematocrit or severe bleeding.
• Avoid giving steroid.
• Do not use antibiotics.
• Do not change the speed of fluid rapidly, ie, avoid rapidly increasing or rapidly slowing the speed of fluids.
• Insertion of nasogastric tube to determine concealed bleeding or to stop bleeding (by cold lavage) is not recommended since it is hazardous.
• What should not be done is as important as what should be done.
• What should be done should not be over done.
Signs of Recovery
• Stable pulse, blood pressure and breathing rate
• Normal temperature ,
• No evidence of external or internal bleeding
• Return of appetite
• Good urinary output
• Stable hernatocrit
• Convalescent stable petechial rash
Criteria for Discharging Patients
• Absence of fever for at least 24 hours without the use of anti-fever therapy
• Return of appetite
• Visible clinical improvement
• Good urine output
• Minimum three days after recovery from shock
• No respiratory distress from pleural effusion and no ascitis
• Platelet count of more than 50,000/mm3
Blood Sample Collection for HI test from suspected Dengue patients
1. In the acute stage: 0-5 days after onset, volume 0.5 – 1.0 ml (Serum specimen S1).
2. Shortly before discharge from hospital: 6-10 days after onset (Serum specimen S2).
3. If possible, 14-21 clays after the onset of disease (Serum specimen S3).
The serum should be separated from the red blood cells and stored frozen before examination. If refrigeration is not possible for keeping blood samples, What man No 3 filter paper discs 12.7 mm (1/2 inch) in diameter may be used. Collect the blood on the filter paper and fully saturate it through to the reverse side. Allow the filter paper to dry in place that is protected from direct sunlight and insects. Place the dried strips in plastic bags and staple them to the laboratory examination request form. Store without refrigeration.
All collected samples should be adequately labeled with name of the patient, their identification number and date of collection.
Indication & preparing patient or family members for possible blood requirement
Indications for whole blood
I. Hemoglobin level 5 gm %
2. Significant bleeding 10% of total blood volume (TBV). TBV of body is 80 ml/kg.
3. Concealed bleeding manifested by Hematocrit drop and unstable vital signs in spite of adequate volume replacement.
Dose of whole fresh blood: 10 ml/kg/dose at a time.
Indication for platelet concentrate
It has been observed that there is very limited role of platelet transfusion. In most of the situation fresh whole blood transfusion is suffice. However it may be required in some special situation. The indication of which may be as follows:
1. Platelet count 10,000 /mm3
If platelet concentrate is not available fresh whole blood may be transfused as per guidelines given under DHF management.
Indication for fresh plasma I plasma substitute
1. Serum albumin 2.0 gm/dl
5. VECTOR SURVEILLANCE AND CONTROL59-61.
Entomological surveillance is used to determine changes in the geographical distribution and density of the vector, evaluate control programme, obtain relative measurements of the vector population over areas and facilitate appropriate and timely decisions regarding intervention. It may also serve to identify areas of high-density infestation or periods of population areas. A number of methods are available for detecting or monitoring immature and adult populations. . ;
Several indices have been described and are currently used to monitor Aedes aegypti populations for dengue virus transmissions. Those related to immature populations include the house index in the percentage of houses infested with larvae or pupae; the container index, in the percentage water-holding containers infested with larvae or pupae; and the Breteau index, the definition of house should be one unit of accommodation and the surrounding premises irrespective of the number of people residing there in.
The abundance of adult mosquitoes is expressed or either the landing rate or the indoor resting density during a fixed period of collection time. Landing or biting collections on humans are a sensitive but labour intensive means of detecting low-level infestations. Rates of capture are usually expressed in terms of landing-biting counts per persion-hour. Resting collections consists of the systematic search for Aedes aegypti, which typically spends periods of inactivity in secluded places indoors such as in closets and under furniture. Resting collection studies performed, with backpack aspirations are an efficient and effective means of evaluating adult densities. Densities are recorded either as the number of adult mosquitoes per house (females, males or both) or the number of adult mosquitoes collected per unit of time.
Wherever larval surveys indicate low infestation (e.g. when the Breteau index <5), ovitraps can be used as a complementary surveillance method, they have proved especially useful for the early detection of new infestations in areas from which the mosquito had been eliminated. Aedes aegypti has a relativity short flight range, but even 100 yards. So a very large number of catching stations are required to provide accurate monitoring of areas at risk143’146. Since this is not usually feasible, the best alternative is to concentrate monitoring on high-risk areas as determined by experience or environmental condition. Special attention should also be given to the evaluation of areas where control activities have been carried out. so that remedial can be implemented if required.
In areas of high human population density, many people may be exposed even if the mosquito house index is low. Distance between houses may thus be of epidemiological significance, especially in areas with single storey dwellings, the population per unit area is likely to be higher and thus survey data for simple-storey and multi-storey dwellings should be kept separate. In tropical countries where there are large areas free of the vector, surveillance against infestation is of paramount importance. Special attention should be given to the surveillance of seaports, airports, other points of entry cemeteries and used-type storage or refreading facilities.
Ports that receive vessels from infested areas should have ongoing inspection programmes. Cemeteries where live or artificial flowers are placed in bases and other containers are important Aedes loci. A good surveillance programme to avoid infestation is much less costly than an eradication or control program that must be established after infestation has occurred.
5.1 VECTOR CONTROL
The most effective means of vector control is environmental management which includes planning & organization, carrying out and monitoring activities for the medication or manipulation of environmental factors with a view of preventing or reducing vector propagation and human-vector pathogen contact.
In Asia and the Americas. Aedes aegypti breeds primarily in man made containers. while in Africa, it breeds both in natural containers such as tree holes and leaf axil and in artificial containers. Control of Aedes aegypti in Cuba and Panama in the early part of 20tn century was based on forms of environment management and many programmes in the Americas are returning to this fundamental tactic. Environmental management is also part of the coinrol measures taken against Aedes albopictus. a secondary vector for the dengue in the pacific Asia and a potential vector following recent infestation in Africa, Southern Europe and America. In 1980. the WHO Expert Committee on Vector Biology and control defined three types of environmental management.
1. Environmental modification- long-lasting physical transformations of vector habitats.
2. Environmental manipulation – Temporary changes to vector habitat as a result of planned activity to produce condition unfavorable to vector breeding.
3. Changes to human habitation or behavior- efforts to reduce human-vector-pathogen contact.
Methods of environmental management
These include the improvement of water supply and storage, solid waste management and the modification of man-made larval habitats.
5.2 IMPROVEMENT OF WATER SUPPLY AND STORAGE
Portable water must be delivered in sufficient quantity and consistency to reduce the use of water storage containers that serve as larval habitats such as drums, overhead tanks and jars. Water piped to households is preferable to wells communal standpipes rooftop catchments and other delivery systems. If storage tanks, drums and jars are required for water storage, they should be covered with tight lids or screens.
5.3 SOLID WASTE MANAGEMENT
Basic rule “reduce, reuse, recycle.” Plastic containers used tires etc should be recycled or disposed by proper incineration in waste transformation facilities. The whole tyres should be buried in a separate area of a landfill to avoid their rising upwards under compaction and disrupting soil cover.
5.4 MODIFICATION OF MAN-MADE LARVAL HABITATS
Common-sense approaches should be employed to reduce the potential for Aedes aegypti mosquitoes to breed in and around human habitats. As for example hollow stems for fence such as bamboo should be cut to the node. Tires and container stored outside should be covered placed in a shed and buckets and other small containers should be inverted if stored outdoors. Roof gutters, outdoor sinks, laundry basins and similar items that can retain water and serve as larval habitats should be drained and kept free of debris. Ornamental pools and fountain can either chlorinated or populated with larnvivorous fish. Theses measures and others will help to reduce or prevent the breeding of vector mosquitoes near humans and thereby diminish the risk of dengue viral disease.
5.5 CHEMICAL CONTROL
Current methods for applying insecticides includes larvicide application, perifocal treatment and space spraying DDT, organophosphate insecticides including fenthion, malathion, fenitrothion and temeplus were used for Aedes aegypti control previously.
5.6 PERSONAL PROTECTION
Personal protection measures have been extensively used in efforts to protect indigenous and rural population against day-feeding Aedes vectors of Dengue. Bed ridden, infants or those who must sleep during the day, tourists and short-term visitors to dengue endemic areas should use commercially available insect repellants. For residents and those staying longer in endemic areas, clothing can be impregnated with permethrin.
5.7 BIOLOGICAL CONTROL
Larvivofous fish and the biocedes bacillus thuringiensis H-14 (BT,) are the two organisms most frequently employed.
5.8 DISEASE SURVEILLANCE & OUTBREAK PREVENTION & CONTROL59-61
Surveillance of Dengue
The objective of a DF and DHF surveillance programmes is the early detection of outbreaks that permit the prompt implementation of control measures, hi order to accomplish this, the factors favoring an outbreak should be monitored. This requires the monitoring of suspected cases of DF & DHF, case reporting and entomological investigation. With modern air travel, a viraemic patient can quickly, move from an endemic to a receptive area .
Following activities, therefore, should be included in a basic programme of DF and DHF surveillance.
1 Fever surveillance
2 Recognition of Dengue hemorrhagic fever
3 Reporting of case to health authorities
4 Aedes surveillance
5 Virological surveillance
6 Development of epidemic contingency places
7 Control of DHF
8 Emergency mosquito control
9 Exchange of information
6. METHODOLOGY
6.1 Type of Study: Cross section Observational study.
6.2 Period of Study: 15th June – 14th August , 2007.
6.3 Place of Study: Medicine Units, Dhaka Medical College Hospital.
6.4 Inclusion Criteria: All patients diagnosed as Dengue Syndrome in Medicine Wards was included in this study.
6.5 Study Population: 90 patients in 5 medicine units were reviewed .
6.6 Ethical Issue: Verbal consent was taken from all patients after thorough explanation.
6.7 Data Collection: Data was collected secondarily from hospital records. A standard proforma and questionnaire was designed and filled to collect data from patients with the diagnosis of Dengue Syndrome and their classification of Dengue fever , Dengue Hemorrhagic fever Grade I , II , III , IV Then management of each patient was reviewed to compare with national guideline.
7. RESULTS
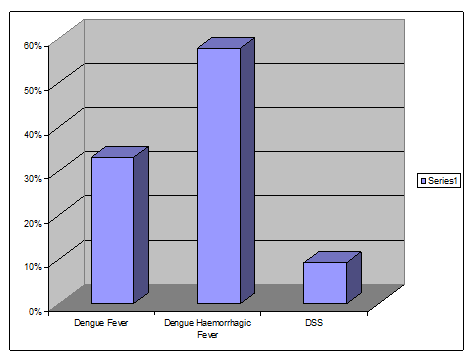
Among the 90 studied cases
• 30(33%) patients were diagnosed as Dengue fever.
• 52(57.7%) patients were diagnosed as Dengue Hemorrhagic fever.
• 8(9.3%) patients were diagnosed as Dengue Shock Syndrome.
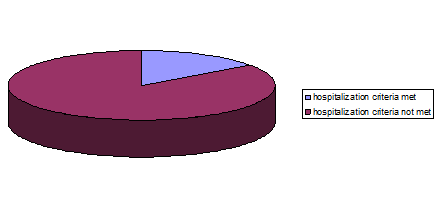
7.1 INDICATION FOR HOSPITALIZATION
Among the hospitalized patients criteria for hospitalization in Dengue fever was not met in 85% patients.
7.2 FLUID MANAGEMENT
Among the 90 studied patients measuring weight before fluid management was done for only 25 patients.
7.3 FLUID MANAGEMENT FOR DHF I AND DHF II
According to national guideline fluid management for DHF I and DHF II, Initial fluid management was
• Inadequate for 4 patients (7%)
• Adequate for 6 patients (11%)
• Excess for 0 (%)
• Could not be evaluated as weight not mentioned for 42 patients (82%).
According to national guideline fluid management for DHF I and DHF II, Subsequent fluid management was
• Inadequate for 0 patients (0%)
• Adequate for 3 patients (6%)
• Excess for 7 (12%)
• Could not be evaluated as weight not mentioned for 42 patients (82%).
7.4 FLUID MANAGEMENT FOR DSS
According to national guideline fluid management for DSS, Initial fluid management was
• Inadequate for 0 patients (0%)
• Adequate for 2 patients (25%)
• Excess for 0 (%)
• Could not be evaluated as weight not mentioned for 6 patients (75%).
According to national guideline fluid management for DSS, Subsequent fluid management was
• Inadequate for 0 patients (0%)
• Adequate for 0 patients (0%)
• Excess for 2 (25%)
• Could not be evaluated as weight not mentioned for 6 patients (75%).
7.5 USE OF ANTIBIOTIC
Among the hospitalized patients with Dengue syndrome antibiotics were
• Used in 9 patients (10%).
• Not used in 81 patients (90%)
7.6 USE OF WHOLE BLOOD
Among the hospitalized patients with DHF and DSS, 6 patients received blood. Criteria for Blood Transfusion were
• Met in 2 patients (33.3%)
• Not met in 4 patients (66.6%)
7.7 USE OF STEROID
Among the hospitalized patients with Dengue syndrome Steroid were
• Used in 2 patients
• Not used in 88 patients
7.8 USE OF PLATELET CONCENTRATE
Among the hospitalized patients with DHF and DSS, 3 patients received Platelet concentrate. Criteria for Platelet Transfusion were not met.
8. DISCUSSION
In 2000 Bangladesh first experienced Dengue epidemic in major cities of the country with more than 5000 people affected and 93 death. But due to implementation of National Guideline of Dengue fever the death toll declined to a minimum in subsequent years.
This study was designed to see whether The national guideline for Dengue fever is still practiced in DMCH medicine wards or not.
A total of 90 Dengue patients were included in the study. Among them 30 patients had Dengue fever, 52 patients had Dengue Hemorrhagic fever, 8 patients had Dengue Shock Syndrome.
85% of the Dengue fever patients didn’t fulfill the criteria for hospital admission where rest of the 15% had associated high risk illness.
Regarding the fluid management measurement of patients is vital but out of 90 patients of Dengue fever only 25 patients were weighted. And rest of the patients were treated without actual measurement of weight.
In DHF Grade 1 and 2 initial fluid management was found inadequate for 7% patients, adequate for 11% patients, and in 82% of patients fluid measurement couldn’t be evaluated as weight was not taken. But subsequently after improvement Fluid was excess for 7% of patients and inadequate for 3% patients. Regarding fluid management National guideline were not followed at least 89% of patients.
Similarly in DSS initial fluid management was found adequate for 25% patients and in 75% of patients fluid measurement couldn’t be evaluated as weight was not taken. But subsequently all of 25% patients were given excess fluid after improvement. So here also National guideline were not at all followed properly.
9 out of 90 patients had received antibiotics where no other cause of antibiotic use were found. The need for blood transfusion and platelet transfusion has declined after implementation of National guideline. This is also reflected in this study, as only 6 patients were transfused with blood but among them 4 didn’t met the criteria for transfusion. And 3 patients were transfused with platelet where any of them didn’t met the criteria for transfusion. Steriod in the form of intravenous Dexamethasone was also used in 2 patients where no obvious cause was found.
9. SUMMERY AND CONCLUSION
Dengue is now established as most wide spread mosquito borne viral febrile illness in human and no doubt has been emerging as a public health problem in Bangladesh. So we need to be prepared to face this menace with a specific target of reducing the case fatality and morbidity. And implementing national guideline would be useful in this regard, as it helped to reduce the mortality in the subsequent years of initial epidemic.
But this study showed National Guideline for Clinical Management of Dengue Syndrome is almost not followed properly in Dhaka Medical College Hospital despite extensive training programs during the Dengue season.
The reason behind this could be all doctors working in the wards are not well oriented about the management of Dengue Syndrome according to National Guideline, Unavailability of weight taking machine during emergency management, Unavailability of Fluid chart for Dengue Management in wards according to National Guideline, or huge workload in admission wards.
As Dengue has become an endemic in this country, it will cause frequent epidemics in the future also. Though the mortality rate is low during endemics but it may turn disastrous if National Guideline is not practiced regularly in the wards.
So the study may be a wake up call to the Medicine wards to make a working plan for dengue Syndrome patients.