Main observation of this thesis is to analysis Effect of Mercerization on Cotton goods in Woven and Knit Dyeing process. Other observation are analysis the management system and get a clear concept on production planning, costing, marketing techniques etc. Thesis also discuss the change of trend, design and style among times and know about how a technical person developed quality of product.
Introduction
None of the imitations of silk has been more widely adopted than mercerized cotton. Mercerization is a process applied to cotton yarns or fabrics which gives to the cotton fiber a silk-like luster, a somewhat greater strength than that of ordinary cotton, and a greater affinity for dyes. Mercerized cotton is at the present time a direct competitor of silk in a great number of ways, both as an imitation and as a substitute. Its qualities are so excellent, however, that were it not for its value as a silk substitute it would still rank above ordinary cotton in its own right. Mercerized cotton has proved itself a most desirable addition to the textiles.
HISTORY OF MERCERIZATION
John Mercer.-The process of mercerizing cotton was discovered about 1844, by an Englishman named John Mercer, but he thought so little of his discovery that he took no patent on the process until 1850. At the time of his invention, he was a chemist in a large calico printing plant. His name is well known in textile chemistry. Besides mercerization, he invented several styles of calico printing and prepared for the first time a sulphonated oil (known as Turkey red oil) ever since used in producing certain fast dyes. He was the inventor of the blue-print photographic process, and also of several medical or pharmaceutical preparations.
Story of mercerized cotton.-Samples of mercerized cloth were exhibited at a world’s fair in London in 1857 and attracted considerable attention; but the cost of the chemicals used in mercerizing was then so high that the process seemed hardly feasible. Mercer, who died in 1866, was therefore in no way benefited by this valuable invention. Not until the later eighties did mercerization become practical, and then for two reasons. First, certain improvements were discovered in the methods of mercerizing; and, second, the cost of the needful chemicals had considerably lessened since Mercer’s time. By Igoo mercerized cotton was in extensive demand and the annual production and consumption have climbed every year since then. Its use is now widespread in a great number of fabrics, as the sole textile in some cases, as the warp in others, as filling in still others. In almost every sort of fabric in which silk is used, mercerized cotton is also employed. Its cheapness permits its use in a number of things for which silk would be impracticable because of prohibitive cost.
Why Mercerization?
Mercerized cotton is sometimes referred to in the crafts as pearl or Pearle cotton. It is cotton yarn or fabric which has been put through a series of processes, primarily to increase luster. The added desirable water handling properties gained are a secondary bonus.
Cotton fiber grows in a bowl; each fiber is produced from an individual seed (about 5,000 altogether) in the base. The fiber starts out as a projecting hollow sheath and each night a new layer of cellulose is laid down on the inside of the sheath until about thirty layers are built up. At this point the fiber is like a solid cylindrical rod having a central lumen or canal pointing to the tip consisting entirely of cellulose. When the boll bursts and exposes these fibers to sun and air they dry up and collapse, becoming flatter and ribbon like with alternating left and right spiral twist every two or three turns. This is cotton fiber in its original state.
Through the ages countless attempts have been made to alter the fiber, sometimes with a specific end use in mind and other times just as pure research. In 1851, John Mercer was granted a British Patent for work he had done pertaining to cotton, linen and other vegetable fibrous materials that in effect caused certain changes in the character of the fiber when subjected to caustic soda, sulfuric acid, and/or other chemicals, etc. He went on to list a number of these changes, one of which was that caustic soda caused the fiber to swell, become round and straighten out (but it did not impart any change in luster). At the time Mercer introduced these processes, the British cotton trade showed no interest in any of it and it all sat in obscurity for about forty years. In 1890 Horace Lowe was granted a British patent in which he claimed that by applying Mercer’s caustic soda process to cotton yarn or fabric under tension a resultant high luster (a result of the light reflection off the smooth, round surface) was imparted to the fiber. It became an overnight success and revolutionized the cotton industry. The rest is history.
INTRODUCTION
Mercerization is a process in which textiles (typically cotton) are treated with caustic soda (NaOH) solution to improve properties such as fiber strength, shrinkage resistance, luster, and dye affinity. The fabric also has smoother and more lustrous look when mercerized under tension. The caustic actually rearranges the cellulous molecules in the fiber to produce these changes. Higher-end fabrics may be double or triple mercerized for added benefits .effective mercerization requires attention to variables such as caustic soda strength, dwell time (feed rate), temperature, and neutralization. The feed rate of then fabric may also be limited by its strength and weight and is usually run at 80 to 120 yards (73 to 110) per minute.
Under the action of concentrated alkaline solutions chemical, physico-chemical and structural modifications of cellulose take place .chemical reactions lead to the formations of alkali cellulose, physical reactions , to intensives swelling of fibers and structural reactions, to a change in the arrangements of units in the cellulose macromolecule.
Concentrated solutions of caustic soda cause considerable swelling of cotton fiber, the changes in cellulose physical properties being irreversible. When the fiber swells, its volume undergoes considerable changes; at maximum water absorption, the cross section of cotton fiber is increased by 40 to 50% with inconsiderable increase in length (about 1 to 2%).
The finishing operations performed on weaving fabrics include all the operations carried out to provide a fabric with those properties that the customer desires after the fabrics leave the textile factories. With the modern textile finishing operations that are applied today, it is possible to provide cotton fiber with a structure similar to the superior properties of synthetic fibers. One of the finishing operations that change the physical and characteristic properties of cotton fiber and the most important of these is mercerization. Mercerization is the treatment of cotton yarns, fabrics and knit goods with cold, strong caustic soda liquor under tension. Mercerizing is an important operation for cotton finishing achieving a resistant silk shine and good handle without all the fundamental processes being understood. Cross section of mercerized cotton fibers becomes larger and assumes perfectly circular forms as shown in Figure 1. In mercerization, the process is based on treating the threads or fabrics with a caustic soda (sodium hydroxide) solution of 15 – 20%. The threads or fabrics are rinsed a number of times after it has been subjected to sodium hydroxide. The fabrics are subjected to tension on a stenter on which most of the caustic is removed with hot water and then the remainder of base is neutralized in cold acid bath. Then the remainder of the acid is removed from the fabric by washing. The process is continuous. The threads or fabrics are subjected to tension during the finishing process for conventional mercerization. Good results are obtained through proper saturation, sufficient tension and complete washing. In addition, the same results can be obtained for knitted products in the shape of a tube through special equipment.
OBJECTS OF MERCERIZATION:
- Increase tensile strength.
- Improved hygroscopicity.
- Improved dye affinity.
- Improved smoothness.
- Improved luster.
- Improve wear resistance.
- Improve light resistance.
- Improved dimensional stability & physical compactness.
- 20-30% dye and chemical save while dyeing after mercerizing.
- Increase chemical reactivity at low temperature.
AT WHICH STAGE CELLULOSE CAN BE MERCERIZED?
Mercerization is possible
- On greige goods
- After desizing
- After desizing and scouring
- After bleaching
- After dyeing
THE REACTIONS THAT TAKES PLACE IN MERCERIZATION:
Since concentrated sodium hydroxide (operational solution that includes chemicals) is used in mercerization process, the reactions that take place with the cellulose fibers are intermolecular reactions. That is, the sodium hydroxide that is concentrated this much, penetrates inside the micelles (crystallites) and a structure called hydrate cellulose emerges.
Cell – OH Cell – OH + NaOH Cell – ONa + H2O Cell – ONa + H2O
75% 25%
Sodium hydroxide reacts with the hydroxyl groups inside the macromolecule in such a way that it either produces sodium cellulosate or it links to the molecules through the pulling forces. Although both of them take place in mercerization, 75% is on linking’s side through the force of pull.
PHYSICAL CHEMICAL CHANGES THAT OCCUR DURING MERCERIZATION:
When cotton fiber is brought into aqueous solution of sodium hydrose of 15°Tw (18%), the cellulose beings to swell immediately and in a few seconds the hair/ fiber is elliptical in X-section. On further swelling, the section rounds off and the major axis of the ellipse is at least 25-30% greater than the fiber width of the corresponding collapsed fiber. The cellulose of the wall swells inwards until the lumen is partially eliminated. These charges are shown in the following figure
When the fiber is transferred to the water and well washed. Shrinkage beings (stage 6) and on drying at room temperature a further and final shrinkage occurs (stage 7). During the last three shrinkage proceeds uniformly towards the center and the lumen does not recover its original size.
Higher strength is obtained after the process sequence due to:
- Molecular orientation increase.
- Length wise shrinkage of fabric occurs which minimize the weak links in the fiber.
Higher absorbency obtained due to:
- NaOH acts as decrystallinity agent which causes increasing amorphous region.
- Crystal lattice is altered and tends to increase absorbency.
Dye affinity increase due to:
- Increase amorphous region.
- Crystal lattice is altered.
- Available –OH group increase which contribute dye affinity.
Luster increase due to:
- Kidney shaped becomes almost circular (oval shaped) in X-section.
- Almost 200 convolution/ ribbon form/natural twist found in one inch.
- Deconvolution occurs or convolution twist becomes less.
- Increase transparency and reduce surface roughness.
MATERIALS
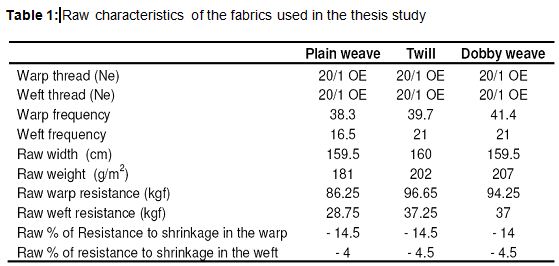
In this study, fabrics, that were woven by using 20/1 Open-end 100% cotton thread and three different weaving pattern, were chosen. These fabrics have medium weights and approximately same construction. The characteristics of these fabrics are given in Table 1. Weave reports of the fabrics used in this study are shown in Figure 3. The machines and equipments used in the processes were as follows:
- Burning and desizing machine in which the operations of burning and desizing are combined.
- Combined bleaching machine that makes hot bleaching and also has 2 prewashing and 4 final washing chambers.
- Mercerization machine that has stabilization section after the caustic chamber, chain frame and a 5 final washing chamber.
- Drying machine with contact drying system in which exit humidity control is carried out with Pleva equipment and which consists of 20 cylinders that work with steam.
- Pad-batch machine. Painting machine with a single tub in which the fabrics are made to absorb the paint and chemicals according to cold pad batch method.
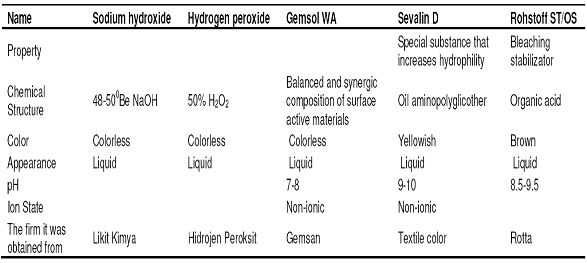
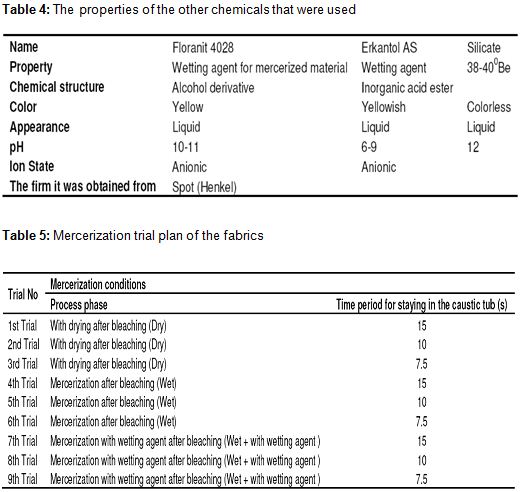
- Chemistry laboratory and beaker, measuring tape, pipette, titration apparatus, etc. for chemical tests. For physical tests, SDL strength test equipment, fully automatic washing machine and drum dryer were used in the product test laboratory. The properties of the chemicals that were used in desizing and bleaching processes and other auxiliary chemicals were given in Table 2, Table 3 and Table 4, respectively.
METHODS
Firstly, burning and desizing processes were applied on the fabrics. Then pre-finishing was carried out on the fabrics by applying the conditions and prescriptions indicated below before the mercerization process. The processes of burning and desizing were done with burning-desizing machine in which a multifunctional burning machine is combined with a tub where the fabric is made to absorb the desizing solution. The technical data and prescriptions are as follows:
Prescription : 2.5 g/l Torozym NT, 2 g/l Schnellnetzer KE, 1 g/l
Emulgator BE-O, 1 g/l Entlüfter BK.
Speed : 70 m/min
Table 2: The properties of the chemicals that were used in removing dressing
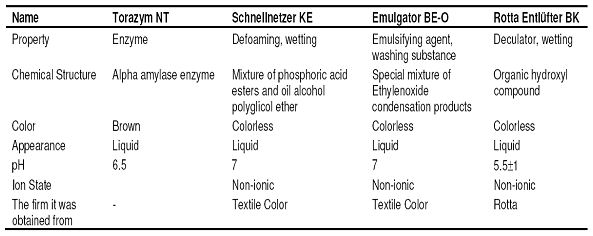
Table 3: The properties of the chemicals that were used in the bleaching process
Burner position : Vertical – double side
Burner distance : 8 mm
pH : 6 – 7
Temperature : 60 – 65°C
Holding : It was held and continuously rotated in the dock for 6 h.
In the textile enterprise, the fabric was made to absorb the prescription above in order to be able to make it capable of dissolving in the water that has sizing which is in those fabrics that have been sized with the CMC sizing substance. It was inserted into a rotation machine for balance and to prevent it from filtering on one side. It was bleached after the rotation period of 6 hour ended. Bleaching process was carried out in a Mega-Bleach, which has two pre-washing Chambers, a Flexnip in which the solution is made to absorb to the fabric, a steam chamber which is appropriate for accumulation type working and four washing chambers at its exit. The technical data and the prescription are as follows:
Prescription : 35 ml/l H2O2 50%, 15 ml/l NaOH 480Be, 2 ml/l Gemsol WA, 2 ml/l Rohstoff ST-OS, 1 ml/l SevalinD.
Speed : 60m/min.
Steam chamber : Accumulation type
Holding: 20 minutes in saturated steam at 98°C the temperatures of the pre-washing and final washing chambers were fixed at 90 – 95°C. The fabrics that were taken from the rotation station were passed from the prescription and working conditions above in the bleaching machine and they were bleached. Then they were dried or mercerized according to the process stage. They were dried in the drying machine, which works with contact drying method and which consists of 20 cylinders that are heated with steam. The exit humidity of the fabrics was measured with humidity measurement device in a controlled way. The technical data are as follows:
Speed : 40m/min.
Temperature : In cylinder drier machine at 140°C.
Humidity : (8 ± 2) %
The fabrics were mercerized after the drying process. They were mercerized according to the plan indicated in Table 5 by using fabric mercerization machine at 60°C and 30°Be NaOH. The fabrics were applied with 3% tension along the warp and mercerization was carried out by stretching the +1 cm width at the needle frame section after the stabilization part along the weft. The fabrics were painted with reactive dyestuff after the mercerization process. The fabrics were made to absorb the dyestuff and chemicals in pad-batch painting machine and which works with single dipping and wringing method, and they were rotated during the fixed period in order to avoid filtration at the rotation stations. The technical data and the prescription are as follows:
Prescription : 14.8 g/l Cibacron Blue CR, 5.6 g/l Cibacron Deep Red CD, 12.1 g/l Cibacron yellow CRG, 1 g/l Erkantol AS, 4.8g/l Caustic 48 0Be, 50 g/l Silicate.
Fixed period : After the dyeing, they were held by rotating at the dock for 14 h. After fixing, the fabrics were washed with hot-boiling water and then dried at 120°C.
THE PLAN OF THE TRIALS TO BE APPLIED ON THE FABRICS IN THE MERCERIZATION MACHINE
The fabrics that have the properties in Table 1 were passed from the mercerization machine according to the plan below and they were tried. Mercerization details of these fabrics were given in Table 5. At 1st, 2nd and 3rd trials, the fabrics were dried after the bleaching process before the mercerization and entered into the caustic chamber as dried. At 4th, 5th and 6th trials, they entered the caustic chamber as wet without being dried after bleaching. As for 7th, 8th and 9th trials, they entered the caustic chamber as wet without being dried after bleaching and wetting agent of 5 g/l was given to the caustic chamber.
After the finishing processes, the sample fabrics were applied the dimension stability test in order to observe the change in their dimensions. Six measurements were done on each sample according to the standard of BS EN 26330 and the average of them was taken. Household type washing machines indicated in standard of TS 5720 EN ISO 6330 were used in the measurements. In the measurements, the fabrics were marked with permanent marker with 35*35 cm gaps (in the direction of wefts and warps) and they were washed in the washing machine at 60°C. After washing they were dried in drum drier and their shrinking percentage values were measured. The results of resistance to shrinkage obtained in this study were analyzed with SPSS 12.0 statistical data analysis program in the computer and their statistical significance was calculated. Two-way ANOVA test was used in the statistical analysis. 5% margin of error (95% confidence interval) was taken as a basis in all the calculations and the results that are upper than P=0.050 value do not have an effect that has statistical significance. The results that are smaller than this value have a statistically significant effect.
RESULTS AND DISCUSSIONS
In dimension stabilization, what is desired is not high swelling in the fibers, but their homogeneity, because the fibers at the outside of yarns swell more than the fibers at the inside of yarns in mercerization at low temperatures and internal tension emerges as a result of the difference between the swellings inside and outside which has a detrimental effect on stabilization.
Table 6: Results of SPSS analysis related to resistance to shrinkage in the warp (Tests of between-subjects effects)
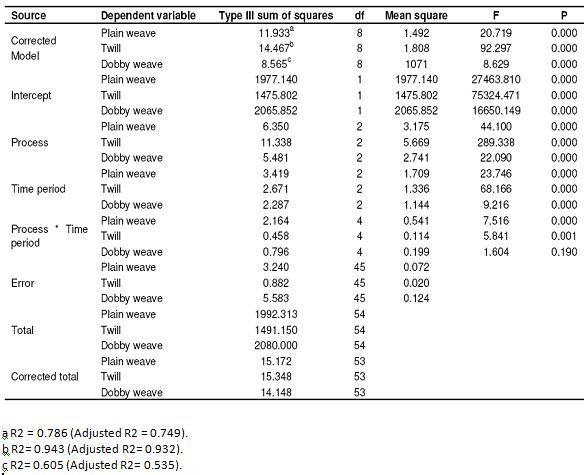
A homogeneous swelling is produced in mercerization at 60°C, but the shrinkage in the fibers changes with the changing of the application time, thus stabilization will also change. As seen in Table 6, all the P values of plain, twill and dobby weave fabrics occurred 0.000 in terms of statistical significance in resistance to shrinkage in the warp except for the process time interactions of the dobby weaving fabric. The process, time period and process time period interactions have a statistically significant effect on the resistance to shrinkage of the warps of the fabrics except for the process time interactions of the dobby weaving fabric. However the P value of dobby weaves fabric appeared to be 0.190, which is higher than 0.050 in the process time period interaction, so process time period interaction does not have a statistically significant effect on the resistance to shrinkage of dobby weave fabric. As seen in Table 7, the P values of plain weave, twill and dobby weave fabrics in the resistance to shrinkage of the weft appeared to be 0.000. Process, time period and process time period interactions have a statistically significant effect on the resistance to shrinkage of the weft. As seen in Table 8, 50 – 60% improvement was seen in the direction of warp compared to the raw fabric. We see the best results at the conclusion of the trials with dry process. The reason for that is that, they gather a little bit in drying.
Table 7: Results of SPSS analysis related to resistance to shrinkage in the weft (Tests of between-subjects effects)
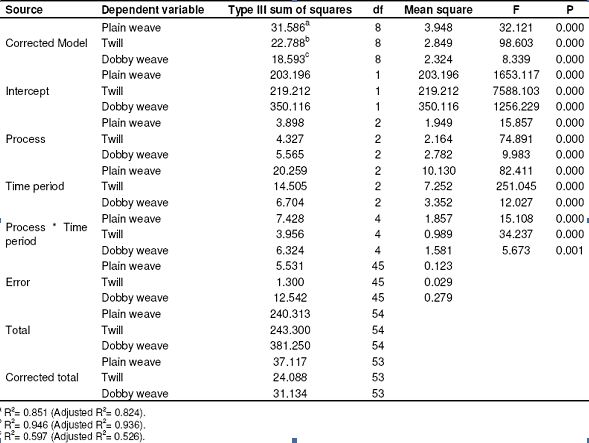
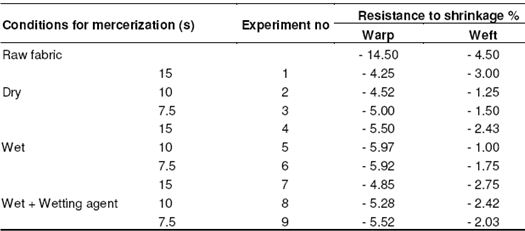
The resistance to shrinkage values gave better results as the time period for application increased in the direction of the warp. 25 – 75% improvement was observed in the direction of the weft depending on the time period. We observed the best results at the conclusion of the trials with wet process. Since the shrinkage in the fibers increase as the time period increases, the resistance to shrinkage in the weft appears to be higher. Changes in the resistance to shrinkage in the directions of warp and weft in plain weaving fabrics depending on process time period are shown in Figure 4 and 5, respectively. As seen in Table 9, 60 – 70% improvement was seen in the direction of the warp compared to the raw fabric. We observed the best results at the conclusion of the trials with dry process.
Table 8: Resistance to shrinkage in fabrics with plain weave
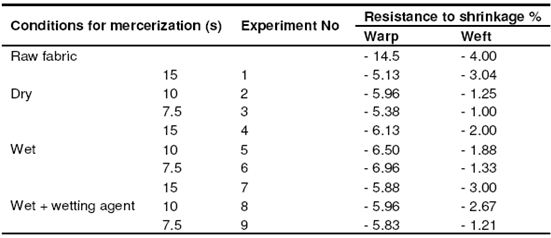
The reason for that is that the fabrics gather a little bit in drying. Depending on the time period, the resistance to shrinkage of those fabrics whose application time period is higher appears to be lower. In the direction of the weft, an improvement of 25 – 75% was observed depending on the time period similar to the plain weave. We observed the best results at the conclusion of the trials with wet process.
Table 9: Resistance to shrinkage in twill fabrics
Since the shrinkage in the fibers increases as the time period increases, the resistance to shrinkage in the weft appears to be higher. Changes in the resistance to shrinkage in the directions of warp and weft in twill fabrics depending on process time period are shown in Figure 6 and 7, respectively.
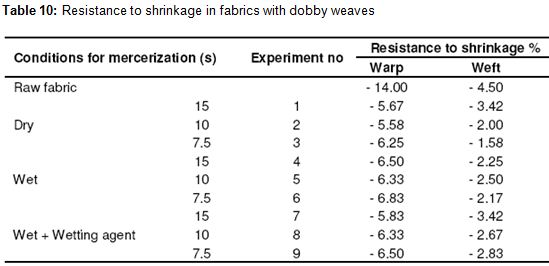
As seen in Table 10, an improvement of 50 – 60% was seen in the direction of the warp compared to the raw fabric in the dobby weave. We observed the best results at the conclusion of the trials with dry process. The reason for that is that the fabrics gather a little bit in drying. No change was observed in the direction of the warp depending on the time period. Changes in the resistance to shrinkage in the direction of warp and weft in dobby weave fabric depending on process time period was shown in Figures 8 and 9, respectively. In the dobby weave, an improvement of 25 – 65% was observed in the direction of the weft as it was observed in plain weave and twill. We observed the best results at the conclusion of the trials again with wet process. Since the shrinkage in the fibers increases as the time period rises, the resistance to shrinkage in the weft appears to be higher. This clarity was not seen in the wet process and values close to each other were observed. In general, the dried fabrics provide better stability in resistance to shrinkage in the warp, but this difference disappears when we think of the effect of drying. That is, there is no improvement in the resistance to shrinkage of those fabrics that are dried with an additional process. Moreover, using a wetting agent did not improve the stability. In the direction of the weft, there was a change depending on the time period of holding. This dependency is such that the resistance to shrinkage values increases as the period of holding increases. The faster the processes of mercerization, the better are the results of stability in the direction of the weft.
RESULT OF THE THESIS:
It was observed that three different fabric samples processed at three different time and conditions indicated the best unshrinking values under dry mercerization conditions and decreased shrinkage values on warp direction with increasing of processing time. It was obtained that shrinkage values on warp direction were greater than those of weft.
MERCERIZATION PROCESS
YARN MERCERIZING:
Yarn is mercerized in two ways:
- Hank form
- Continuous sheet (warp) form.
YARN MERCERIZING IN HANK FORM:
Machine description:
- Two guide rollers are arranged parallel and horizontally or vertically.
- The dia of each roller is 4”-6”.
- One of two rollers is geared to drive anticlockwise or clockwise.
- The distance between the two guide rollers can be altered according to the size of hank.
- Synthetic rubber coated padding roller is placed on guide roller, mounted on ball-bearing to avoid any drag of slip of yarn.
- Two trays are used in the machine where one is moveable tray containing mercerizing solution.
Process sequence:
- The yarn of hank form is placed on the guide rollers whilst they are close together.
- The yarn is then impregnated in the through containing caustic soda solution.
- Impregnation is maintained up to half of the mercerizing rollers.
- The yarn rotates in the alkali solution and stretched the guide rollers and at the same time squeezing rollers the yarn to ensure penetration.
- Impregnation time approximately 3 minutes and then allow tray is covered.
- The yarns are washed by means of spraying unit and squeezed. The first washing is with hot water and then cold water.
- The washed yarn is treated with .002% H2SO4 to remove alkali.
- The stretching is carried out not to exceed an elongation of 3-5%with reference initial length.
- Finally the rollers are brought close together to facility the removal of stretched yarn.
Precaution:
- The yarn must be mounted evenly on the rollers.
- The temp of alkali will be bellow 15°C.
- Tension variation must be reduced.
YARN MERCERIZING IN CONTINUOUS SHEET (WARP) FORM:
Machine description
- A series of boxes as many as eighteen are used.
- There are arrangements of squeezing rollers are guide rollers in each boxes.
- The guide rollers are of 12”in dia made of brass or iron.
- The squeezing rollers are of 10” in dia, 36”in length and are covered with rubber.
- The squeezing rollers are driven at constant speed and the pressure is applied by means of weight and lever.
Process sequence:
- The warps first pass through 2-4 boxes, where they are boiled in a suitable solution of dilute caustic soda and T.R.oil sequestering agent may be used.
- The liquor is usually heated by closed steam coils.
- The warps then passed through1-2boxes, containing cold water for cooling and rinsing.
- Then warps are squeezed and pass through3-4boxes containing caustic soda solution of mercerizing strength.
- Mercerization time 3-5 minutes.
- Penetration is assisted by the rubber coated rollers which determines the amount of tensiono0n the yarn.
- Then 3-0 boxes are used for washing-
- In 2-4boxes, hot washed at60°C temp
- Last 2 boxes are used for cold wash.
- Small quantity of acid is used for neutralization.
FABRIC MERCERIZING PROCESS:
There are three types of mercerizing machine-
- Pad chain type machine.
- Chainless type machine.
- Pad-chainless type machine.
PAD CHAIN MERCERIZING MACHINE:
Machine description:
- A high speed pad chain mercerizing m/c capable of handling fabrics like poplin and at the same time producing a high degree of luster.
- M/C compromises two power full 3-bowlmangles followed by a heavy pattern clip stenter machine fitted with washing and caustic recovery arrangements and souring and washing cisterns.
- The mangles are of open type, enabling the bowls to be easily changed.
- Each mangle is arranged to provide either a single or double immersion in the caustic liquor and two squeezes.
Process sequence:
- The scoured and bleached cotton fabric is passed through a padding mangle containing 55°-65°Tw the NaOH solution at 18°C for 2-3 min with tension.
- Then padded fabric is squeezed with squeezing roller.
- Then fabric is washed with hot water at 70°Cin proper tension
- Then the fabric is washed with cold water in two times.
- The washed fabric is washed with 1°Tw H2SO4 for removing of the remain caustic soda.
- Then the fabric is dried washing in cold water.
CHAINLESS FABRIC MERCERIZING MACHINE:
Machine description:
- No need of padding mangle or chain (stenter)
- Fabric is mercerized in tension.
- No arrangement of pressure or squeezing.
- Wetting agent is used in caustic soda solution.
- Tension pulleys are present due to variation of tension.
- For light fabric, 45-65lbpressure is used and for coarse fabric 250lb pressure is used.
Process sequence:
- The fabric is entered in the bath of caustic soda solution with two down rollers.
- Simultaneously, caustic soda is dropped on two lifted roller with two pipes. So that the impregnation becomes properly.
- The fabric advances 5yds in contact with other rollers so that caustic soda can penetrate perfectly in fabric.
- Then the fabric is passed through pressure mangle and fabric advance of 8yds, in this time, fabric is mercerized and 40-60time required.
- Then the fabric is washed with hot water at 70°C by some rollers by spraying or counter consent process.
- Then the fabric is washed properly with cold water.
- In this machine 9-26yds fabric is mercerized.
- Simultaneously two fabrics can be mercerized.
GREY MERCERIZATION:
If mercerization of material (yarn/fabric) is carried out in grey state i.e. without scouring and bleaching after desizing it is called grey mercerization.
In grey mercerization, a large amount of impurities find their way in mercerization liquor.
The causes for carrying out grey mercerization are-
- It is observed that a softer handle is obtained on goods which are mercerized in grey state compared with mercerized after bleaching. So for obtaining softer handle mercerization is done.
- If maximum tensile strength is to be maintained, it is saber to mercerise before bleaching i.e. in grey state.
- Only some chemicals are required for mercerizing.
- The process is easy.
HOT MERCERIZING:
New process and machinery based on discoveries concerning mercerization were described by I. Rusznak and C. Duckworth (proc. 10th IFATCC congress, Barcelona, may 1975) and referred to by C. Duckworth and L.M. wrennal (J. soc. Dy. Col. 1977. P. 407) the basic principle of this process is described by the following sequence:
- Saturation with caustic soda solution of mercerizing strength, preferably under relaxed conditions at the boiling point of the caustic solution.
- Controlled hot stretching following saturation.
- Controlled cooling of the stretched fabric.
- Traditional, tension controlled washing (chain or ahainless)
- Traditional final washing (in multibox washer).
In this process the penetration of caustic soda into the fabric and fibers is extremely rapid, through and uniform. Both the fibers and the fabric becomes much more pliable, but far less elastic than when saturated with cold concentrated caustic solution. Shrinkage of the fabric is much less than that occurring in the cold process. If necessary, the fabric can be considerably overstretched to get improved luster, tensile strength and dimensional stability.
What are the benefits of Hot Mercerization?
During hot impregnation the lye penetrates faster and more evenly into the yarn core. Swelling therefore does not take place only on the fabric surface. As Compared with cold impregnation the swelling behavior is considerably better and has a positive influence on the dimensional stability. The more uniform swelling gives, with dyed goods, a more equal appearance. With the wet-on-wet method the exchange factor is increased thus permitting a shorter impregnation zone than with conventional processes. In addition, the lye volume is kept very low. The lye concentration is simple to control. The lye bath has been kept purposely small and under it there is one single lye tank integrated into the machine.
The process lye is continually circulated and the temperature and concentration monitored. The automatic lye control permits the addition of fresh lye, water or recovered lye of 20 to 40 °Bé from recycling. Reaction heat is emitted when mixing the process lye. In hot mercerizing this is utilized, while in cold mercerizing it causes an energy consuming lye cooling process. Squeezing to lower lye content is possible with hot mercerizing. The lower lye consumption provides savings in water and steam at lye extraction.
This is achieved by objective selection of the process parameters such as:
- Lye temperature
- Lye concentration
- Lye reaction time
- Fabric lengthwise and crosswise tension
The thus optimized process provides the best mercerizing effect with savings in lye, water and steam. In comparison with other mercerizing ranges the small lye volume permits very fast changes in concentration and, therefore, short reduction interruptions. No lye losses occur at changes. Therefore change over to a lower lye concentration is profitable in addition to the covering of immature cotton.
Savings of lye, washing water and steam are money for your account.
Instead of 45–50 seconds as with cold mercerizing, the same effect can be obtained in only 25–30 seconds with hot mercerizing. In order to keep width contraction as low as possible, the fabric is kept under constant tension during the reaction phase. No selvedge to middle differences occurs with the DIMENSA. In the retention compartment of the DIMENSA the proven chainless principle with lifting top rolls is employed. The retention compartment is not flooded. On the DIMENSA MS the cloth is pinned onto the integrated stenter at the end of the retention compartment. On the DIMENSA ML the retention zone consists of a chainless reaction compartment only.
To summarize the benefits of hot mercerization:
- Faster caustic soda penetration speedier swelling
- High swelling uniformity no ring mercerizing
- Better dye penetration
- Smoother cloth appearance
- softer handle
- Shorter impregnation times
- No cooling needed for mercerizing
- Maximum squeezing action at in feed squeeze
- Lower investment cost through shorter impregnation sections
- Further positive features of tensioned mercerizing are:
- Orderly orientation in the crystal areas
- Removal of fiber unevenness
- increasing the number of microspores
- Unification of the pore size
- And from this result:
- Better residual moisture
- Better luster
[Comparison of Hot & Cold Mercerization]
- Better dyeing behavior with an increased dyestuff yield
- Better handle
- Coverage of immature cotton
SODIUM HYDROXIDE RECOVERY:
Essentials mercerization involves treating of cotton material with strong NaOH solution and washing it off from the material. This gives a large volume of dilute NaOH solution, which cannot be discharge into the drain for economy and pollution point of view. But suitable means it is possible to recover/ re-use 90-95% NaOH used in mercerizing. For example wash liquor may be used (1) for the preparation of NaOH solution (for use in bleaching) by passing chlorine gas into it, (2) in kier boiling, (3) in dyeing vat dyes of cotton, (4) in mercerizing itself by concentrating (evaporation of water in evaporators). Contact of the cloth impregnated with strong NaOH solution with air (carbon dioxide) produces sodium carbonate, which hinder swelling (if re-used untreated). This may be eliminated by adding calculated quantities of lime.
Na2CO3 + Ca(OH) 2 → CaCO3 + 2NaOH
The precipitate CaCO3 may be separated by settling and filtration/decantation. If the wash liquor is used in kier boiling the presence of sodium carbonate is an advantage; the only pretreatment necessary is filtration to remove the loose cotton fibers from the solution.
METHODS OF RECOVERY:
NaOH from the impregnated fabric may be recovered by washing using counter current principle and by using steam in a recuperator. The advantage of the counter current principle over co-current principle is that a relatively small volume of wash liquor of high concentration is obtained in the former.
The fabric enters the washing unit (of chain type of mercerizing machine) with sodium hydroxide solution of 55° Tw strength. During its travel in the stenter it meets a constantly decreasing concentration of NaOH solution. When the fabric leaves the unit it contains the solution of less than 10°Tw strength, which does not shrink the fabric; hence it is released from the width tension of the stenter.
After this the residual alkali may be neutralized with a dilute acid, followed by washing with water to free the fabric from acid. Alternatively, the fabric (with about 10°Tw NaOH solution) may be passed through a recuperator where some more NaOH is recovered.
The recuperator consists of a cast iron chamber containing two sets of guide rollers, the top row begin driven. The wash water is feed into the chamber at the delivery end and is taken out at the entry end. Low pressure steam is admitted into the chamber, which impinges on the fabric, gets condense and removes the alkali efficiently. The wash liquor is then sprayed over the fabric above the last wash tank of the mercerizing machine (stenter). The fabrics leave the recuperator through the nip of a pair of squeeze roller.
THE CONDITION OR CONSIDERABLE POINTS FOR MERCERIZATION
- Concentration of caustic soda solution.
- Tension
- Time period.
- Mechanical effect
- Tensile strength
- Staple length
- twist
- Wash thoroughly
- Wetting agent
- Fabric construction.
EFFECT OF MERCERIZING CONDITIONS:
Temperature:
Temperature has a great effect on swelling Optimum swelling takes place at around 15 – 20°C (Ideal temperature 15°C). However, the swelling speed of fiber at low temperatures falls due to the high viscosity of caustic soda. The heat that emerges during the reaction prevents swelling and therefore, float is cooled in order to achieve the best mercerization effect. The temperature of the solution must be uniform.
Edelstein investigated the effect of temperature on mercerization in the range of 15-43°C. He found that, the lower the temperature-
- Less concentration of caustic soda need.
- Greater tension necessary to prevent shrinkage.
- Greater dye affinity.
- Slightly higher tensile strength.
- Higher luster.
- Caustic recovery easy.
Luster at different temperature
| Temperature (°C) | luster |
| 7.5 | 76 |
| 17 | 71 |
| 25 | 57 |
| 35 | 40 |
Hot mercerization has been recommended because cotton is largely hydrophobic, contains varying amounts of sizing materials when woven and, since cold caustic mercerizing solutions are viscous, surface actives are at very low levels. The result is that mercerizing causes rapid and extensive shrinkage and swelling of cotton production a compacted surface causing further penetration to become difficult. The cold process under conditions can easily produce no uniformity mercerized fabrics.
It has been reported that as far as luster, tensile strength and dimensional stability are concerned, hot mercerization can produce better results than normal mercerization for three reasons. First hot caustic soda can penetrate the fabric and fiber surfaces thoroughly with the result that a greater proportion of the cellulose may be modified. Second, at elevated temperatures, the concentrated caustic causes the fabric to become more plastic but less elastic causing the fabric to be easily stretched. Last, the stitch/tension variable can be easily set at any desired level, which in turn controls the fabric properties.
When goods are mercerized using hot mercerizing liquors, the goods can be stretched to a greater degree than when they are applied cold. As the fabric is stretched in warp and filling directions, greater tensions produce greater luster and strength with improved dimensional stability.
Hot mercerizing generally produces increased dye sorption as the result of the plastic nature of cotton and its response to tension. However, when goods are processed under tensions which are too great, dye sorption and affinity are decreased because the internal fiber structure is collapsed.
Concentration of caustic soda solution.
- Dye absorption increases with the increasing caustic soda concentration up to 13%.
- Tensile strength increases with increasing caustic soda concentration up to 13%and further slight increases with increasing concentration up to 27%.
- If caustic conc. Increases above 27%, tensile strength falls rapidly.
- Most commonly used concentration is 21-22%.
- 13-15% caustic concentration is applied when dye absorbency is important considered.
- Not full conversion of cellulose if the concentration is less than 13%.
Time period:
It has been determined that when a scoured fabric is dipped into the mercerization flotte, the time required for completely swelling of fibers is 60 s. However this period is getting less with the aid of mechanical effects as is in the mercerization machines. In a good mercerization machine without chain, the time period required for the full impregnation of fibers with the caustic soda flotte is 12 s for dry fabrics and 20 s for wet fabrics. Meanwhile, the role of wetting substances is great in the mercerization of raw fabrics especially. When the work is done without using a wetting substance, the wetting of the fabric would be insufficient even if the mercerization period is increased to 2 – 3 min. For this reason, full impregnation cannot be obtained
Tension:
- Mercerization without tension gives no luster and causes a considerable shrinkage.
- Maximum luster is obtained when the tension is just sufficient to bring the material back to its original dimension.
- Further increment of tension causes-
- No benefit
- May be harmful
- Fabric damage
- Cellulose-I (natural formation) cannot convert fully into cellulose-II (after mercerization formation).
- Dyeing properties increase as mercerization carried out under decreasing tension.
- Impregnation is carried out at relayed condition but washing carried out under tension.
- The tenacity and elastic modulus increase significantly when the mercerizing tension increase.
- Extension at break decrease when tension increase.
Mechanical Effects:
During the mercerization process, the thread or fabric is tightened and the crystals get into the parallel to the axis of the fiber inside of fiber and consequently, the links among the macromolecule chains (H Bridges, Van Der Wall’s forces) increase even more. However, flexibility decreases since the links among macromolecule chains, crystallites and fibrils are even stronger.
Tensile strength:
After mercerization of cotton, its tensile strength increases up to 10-50%. Generally it depends on concentration of caustic soda, temperature, impregnation, fabric structure etc. If temperature and concentration of caustic soda increase up to a certain limit the tensile strength of cotton increases. Tensile strength is higher when caustic concentration is 45-54°Tw and temperature is 17°C.
Tensile strength with temperature:
| Temperature (°C) | Tensile strength (gm) |
| 7 | 302 |
| 17 | 276 |
| 30 | 253 |
Tensile strength with caustic soda concentration:
| caustic soda concentration (Tw) | Tensile strength (gm) |
| 25 | 247 |
| 35 | 287 |
| 45 | 288 |
| 52 | 302 |
| 60 | 282 |
Staple length:
Higher the fiber lengths lower the twist. As a result mercerizing will be better and luster increases.
Twist:
Effect of twist on mercerization is high. If yarn twist is high then alkali doesn’t enter easily. As a result improper mercerization occurs and luster also not well. On the other hand in low twist yarn alkali enter easily and luster will be also good. Generally the range of twist factor is 2.2-3.0.
Washing thoroughly:
- Washing under stretched condition after mercerization is very important.
- In commercial mercerization process, the strong NaOH is washed out of cotton by hot water.
- After washing, caustic soda concentration in the fabric must be less than 8%, otherwise shrinkage occurs if tension is reduced.
- 01-0.5% HCl may be used to neutralize caustic soda.
Wetting agent:
A wetting agent is included in the mercerizing liquor which reduces mercerization time as a result of successful, uniform and rapid penetration of NaOH.
- Commonly 0.5-1% of wetting agent is used.
- For grey fabric, this amount may be 2%.
- In case of scoured and bleached fabric, wetting agent is not important but using 0.1-0.5% gives better effect.
- Cost of wetting agent should be considered before using.
- In industry, wetting agent is referred as mercerizing oil.
Fabric construction:
The type of cotton materials to be mercerized is varied to the point that it is impossible to make many quantities statements about them. The important facts to remember are:
- The primary object of mercerization usually is to produce luster.
- Luster is obtained through the use of tension; and
- From the standpoint of yarn construction, the larger the proportion of fibers laying length wise to the yarn (low twist) the better the luster will be.
Fiber is the fundamental unit in either yarn or in cloth. Having realized this, it becomes obvious that long fine staple cottons are best for mercerizing because they can be combed and spun into yarns having a small amount of twist and the individual’s fibers can lye fairly parallel to the lengthwise direction of the yarn. After the yarn is spun, numerous loose ends of fibers produce from the surface of the yarns. It is necessary then that mercerized yarns and fabrics should be signed in order to achieve greatest gloss or luster.
Mercerization of cotton fabric with low concentration sodium hydroxide solution at low temperature
Generally cotton mercerization is conducted in a mercerizing bath containing high concentration of caustic soda or sodium hydroxide solution such as 20-30%. After mercerization, the alkali waste solution is often treated with an acid for neutralization of the waste treatment process. This project is proposing a possibility of mercerization of cotton fabric with lower caustic soda concentration than 20% through a mercerization at low temperature. Cotton woven and knitted fabrics were mercerized with 10-30% caustic soda solutions at various temperature of 5-40 ํC and were tested for the degree of mercerization, dye absorption, strength, and PH. Fabrics mercerized with 10% solution showed same degree of mercerization as the unmercerized fabric but they obtained better dye absorption ability and higher fabric strength and elongation. While fabrics mercerized with 15-30% solution showed higher degree of mercerization, dye absorption, and fabric strength and elongation than the unmercerized fabric. To obtain an acceptable mercerizing outcome, cotton fabrics need to be mercerized at concentrations not lower than 10%. The recommended mercerizing condition is to mercerize at 15% caustic soda solution at 10-20 ํC for 30 seconds.
WHY WASHING IMPORTANT IN MERCERIZATION?
Washing condition is important after mercerization because the fabric will shrink when washed under slag condition. If washing is not carried out under tension and the concentration of NaOH remains more than 8% after washing then considerable amount of shrinkage of fabric occur. Again if the remaining NaOH is not neutralized that will cause harm to human by hydrolyzing of cellulose. So washing is very important after mercerization and its condition carefully should be maintained.
WHY STRENGTH INCREASE?
Strength increase due to-
- Molecular orientation increase.
- Length wise shrinkage of fabric occurs which minimize the weak links in the fabric.
REQUREMENTS OF GOOD WETTING AGENTS:
- It should posses a high wetting power.
- It should enable the alkali to swell the cotton fiber rapidly.
- Should not be preferentially absorbed by the fiber being treated.
- It should disperse perfectly.
- It should be soluble in caustic soda solution.
- It should not give rise to excessive foaming or turbidity in the solution or deposits on the fiber and machine parts.
- It should not discolor the fiber permanently.
- Its effects of mercerizing liquor should be permanent.
- It must be easily removable.
- It should able to reduce the surface tension of water.
- It should not be injurious to the workers.
- Should not interfere with the caustic recovery from the wash liquors after mercerization.
- Should be available at an economical price.
Generally, there are two types of mercerizing wetting agents-
- Cresylic and
- Non-cresylic
There are not mercerizing agents as erroneously described by some auxiliary manufacturers but are only wetting agents to be used in the mercerizing liquor; sodium hydroxide is the actual mercerizing agent.
MOISTURE REGAIN:
The moisture regain values of cotton yarn mercerized with sodium hydroxide solutions of various concentrationsat10°,18°and 31°C were determined by oven drying method and are given in table
It is seen that the moisture regain of mercerized cotton increases with increase in sodium hydroxide concentration. As in the case of barium activity number, the moisture also increases with decreases in the temperature of mercerization at a given sodium ion concentration. However, at lower concentration at30°C, the treated cotton does not show any barium absorption, but the moisture regain increases, due to the differences in the molecular size of barium hydroxide and water
Table 11: Moisture regain values of cotton yarn mercerized at different temperature
| Concentration of NaOH, % w/v | Moisture | ||
| 10° | 18° | 31° | |
| 5.00 | 6.97 | 6.79 | 6.80 |
| 4.60 | |||
| 10.04 | 7.19 | 7.12 | 7.11 |
| 9.06 | |||
| 16.00 | 7.68 | 7.54 | 7.56 |
| 15.62 | |||
| 19.52 | 8.60 | 8.50 | |
| 19.05 | |||
| 19.55 | 8.54 | ||
| 25.10 | 8.78 | 8.72 | |
| 24.86 | |||
| 25.57 | 8.68 | ||
| 29.75 | 8.89 | 8.78 | |
| 29.26 | |||
| 29.85 | 8.70 | ||
| 33.47 | 8.96 | 8.82 | |
| 33.00 | |||
| 33.80 | 8.71 | ||
Moisture regains values are plotted against the corresponding barium activity number.
It is seen that moisture regains increases almost linearly with barium activity number.
Solutions of sodium hydroxide in the concentration range 16-19 %(wt/vol) appear to be more critical in that a small variation in the concentration causes considerable changes in the properties (moisture regain and BAN) of the mercerized cotton. On the other hand, solutions of sodium hydroxide whose concentration is about 29% (wt/vol), i.e. 52°Tw are not much sensitive.
MERCERIZATOIN (FOR LUSTRE) CAN BE CARRIED OUT IN TWO WAYS:
- By unrestricted swelling
By treating the cotton with NaOH solution, allowing it to shrink to the maximum content following by stretching to the original width (if cloth) or length (if yarn in the hank form)
- Restricted swelling
By treating the cotton under tension with strong NaOH solution without allowing it to shrink and then washing while still in the stretched condition.
THE LUSTER OF MERCERISED COTTON DEPENDS ON VARIOUS FACTORS:
- Cross-section of the fiber.
- Staple length of the fiber.
- Wall thickness of the fiber.
- Concentration of NaOH.
- Temperature of the mercerization solution.
- Percent stretch.
- Yarn construction.
- Yarn twist.
- Doubling of yarn.
- Degree of singeing.
- Application of tension.
- Rate of dyeing.
MERCERIZATION OF COTTON:
Cotton fiber is a long chain polymer of glucose residue.
Two glucose units are considered to give the monomer of cotton. When cotton is treated with caustic soda solution, soda solution is formed which causes swelling of fibers.
Fig 11: Cellulose, the polymer of cotton, with a degree of polymerisation
About 5000 cellubiose units (i. e. n=5000)
The primary and secondary alcohols in the cellulose are acidic in nature. These hydroxyl groups form the basis for the high hydrogen bonding and orientation found in the fibers.
The hydroxyl groups independently dissociate to the extent of alkali sorption. As a result, there is an osmotic pressure increases which causes water to enter the fiber until such time as the osmotic pressure is in balance.
TESTS
Fabrics are generally tested in order to determine the effectiveness of the mercerizing process and these tests are:
- Microscopic examination.
- Benzopurpurine test.
- Sodium hydroxide spotting test.
- Iodine test.
- I. Direct Blue No. 1 test.
The simplest test for mercerized cotton is to determine the number of twisted and untwisted fibers in a yarn bundle. The ratio of the two sets of data may be used to estimate the degree of mercerization.
The Benzopurpurine test is made by boiling samples of mercerized and unmercerized cotton for 30 minutes in a 0.5% solution of benzopurpurine. The samples are washed, dried and compared visually or using a spectrophotometer. The mercerized sample is always more deeply dyed than the unmercerized. However it is recommended that a standard swatch be calibrated so that the actual desired degree of mercerization can be assured.
A simple test can be made by first spotting an undyed fabric with a 30% sodium hydroxide solution. The fabric is then dyed using benzopurpurine. If the fabric is fully mercerized, the spots will not be evident; on the other hand, if the fabric is not mercerized or of a low degree of mercerization, dark spot will be evident.
The Iodine test is done using 20 grams iodine in 100 milliliters of a saturated potassium iodide solution. The fabric is immersed in the solution for three minutes and then rinsed thoroughly. Mercerized cotton is stained bluish black and the unmercerized cotton is remains white. Cotton fibers in the yarn bundle can be counted using a low power microscope. A ratio of dyed to undyed fibers can then be used to determine the degree of mercerization.
The C. I. Direct Blue No. 1 is a simple and direct method for determining the replication of the degree of mercerization. A sample of the fabric is dyed in a 1% OWF dye bath and, after dyeing, is compared with a standard dyeing. If the shade is lighter than standard, the fabric is a lower degree of mercerization; and if darker, it a higher degree of mercerization. The actual degree of mercerization must be established by using other qualified tests.
QUALITIES OF MERCERIZED COTTON:
Loose cotton fibers placed in the caustic solution contract considerably, thus increasing the strength of the fiber. Hence, mercerized cottons, unless stretched too much, are generally considerably stronger than untreated cottons. Not only does the contraction of the fiber strengthen it, but also the thickening of the diameter due to the expansion already described stiffens the structure of the fiber. This much Mercer discovered in 1844 and described in his application for a patent in 1850. But the silk-like luster that we now look for in mercerized cottons had not yet been developed. About i8go some textile makers in Germany were experimenting with the mercerizing process on yarns and woven cloth. It was found, as has already been suggested, that the process shortened the fibers, and consequently caused a noticeable shrinking in the yarn and cloth. This, the experimenters felt, was a disadvantage, and so they concluded that they would attempt to prevent this shrinkage by stretching the cloth and keeping it stretched full length while it was being mercerized. They were successful in keeping the fabric from shrinking, but what was their surprise, on taking the cloth out of the caustic and washing it, to find that it had a beautiful, silk-like luster! The commercial possibilities of this discovery were not overlooked. A description of the process was quickly rushed to the patent offices of all countries, and mercerized cotton, glossy, smooth, and strong, became a big factor in commerce within a few years. Under the low tariff of the Democratic administration from 1893 to 1897, European mercerized cottons were introduced into America, and American manufacturers presently began to produce the same sorts of goods for home consumption. Since 1903 the use of mercerized cotton has increased by leaps and bounds in about the same proportions as silk have increased in American use. When the fashions dictate a great vogue in silks, then mercerized cotton likewise leaps forward. When silks recede slightly, mercerized cotton feels the change also.
USES OF MERCERIZED COTTON:
Mercerized cotton answers many purposes. It is found in such materials as sateens, silkoline, tubsilk, cotton taffeta, linings, dress goods, skirtings, and in embroidery and crochet yarns in its own name. But it is also used in a great number of so-called silk-mixed fabrics, such as silk-mixed mohair, silk-mixed alpaca, silkmixed woolen and worsted figured goods, silk-mixed worsteds for men’s wear, silk-mixed cottons, and so on. It is frequently used in figured cotton damask tablecloths and napkins. Mercerized cottons likewise figure largely in upholstery goods, draperies, curtains, and coverings. Producing crepe effects by mercerization.-The principle of mercerization is sometimes employed to secure crepe effects in union goods, the mercerization attacking only one class of fibers or yarns, as for example the cotton threads introduced at regular intervals in a woolen structure. Such fabrics are called crepons. Between 1895 and 1900 these fabrics had a great vogue in this country. Most of the goods were imported from Germany. How this peculiar effect was obtained was for a considerable time a puzzle Americans until they finally discovered that the drawn up effect, the creping, was due to the shrinking by mercerizing of cotton threads inserted at the time of weaving into the woolen fabric, the wool remaining unaffected by the process.
SPECIAL APPLICATION OF MERCERIZATION:
Not always is the whole fabric mercerized in piece-goods mercerization. Sometimes the cloth to be mercerized is covered with a paste, leaving the cloth exposed only in certain places in the form of figures. In this condition the cloth is immersed in the caustic bath with the result that only the open figures are mercerized, the protected portions remaining plain cotton. The possible variations in finish may be made even more numerous by the dyeing process. Colors may be applied which dye the ordinary cotton faintly while giving the mercerized figures a very full, deep color. Another common method of part mercerization is by mercerizing the cotton cloth in stripes. This gives the seersucker effect. Several other similar types of manipulation are possible, although of interest mainly to the textile manufacturer.
MERCERIZATION MACHINES:

MAIN TECHNICAL FEATURES AND ADVANTAGES
- The machine is suitable to any kind of woven fabric (heavy, light, 100% cotton, linen, blends)
- High flexibility to treat small and medium fabric batches (production speed up to 35 – 40 mt/ 1’).
- The space required for machine installation is reduced to the minimum and the costs of investment are lower compared with the machine working with chain
- Energy saving
- Low cost of maintenance
- If put in line with a continuous washing range, the mercerizing machine does not require any extra maintenance
- Excellent results with possibility to adjust the temperature and the concentration of caustic soda as pleased according to the results required
- Possibility to adjust the tension of fabric in warp while in weft, during the impregnation the fabric is kept in tension by the compression of rolls
Features
SANDO’s mercerizing machine with a clip-type stenter is capable of applying stronger tension on both warps and wefts compared to chainless types. This feature promotes closer to perfect mercerization, resulting in better size stability and drastic improvement in quality of dyeing, as well as excellent luster.
CONCLUSION:
None of the imitations of silk has been more widely adopted than mercerized cotton. Mercerization is a process applied to cotton yarns or fabrics which gives to the cotton fiber a silk-like luster, a somewhat greater strength than that of ordinary cotton, and a greater affinity for dyes. Mercerized cotton is at the present time a direct competitor of silk in a great number of ways, both as an imitation and as a substitute. Its qualities are so excellent, however, that were it not for its value as a silk substitute it would still rank above ordinary cotton in its own right. Mercerized cotton has proved itself a most desirable addition to the textiles.
Study showed that:
- Changes in dimensional stability when considered values for both alkali concentration and temperature were low is very little
- Increase in alkali concentration had a continual positive effect for the samples mercerized at 20°C. But, for the samples mercerized at temperature higher than 20°C increase in alkali only up to 240 g/l improved dimensional stability.
- At the temperature more than 20°C alkali concentration of 300 g/l shows destroying effects when bath temperature was 6020°C and 8020°C.