Acid Rain – an environmental problem
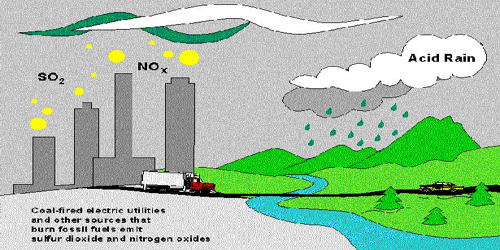
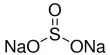
Acid rain occurs when harmful gases such as carbon dioxide, sulphur dioxide, and nitrogen oxide react with rain. It describes any form of precipitation that contains high levels of nitric and sulfuric acids. This type of rain damages trees, crops, and buildings. Industries and power stations release a lot of sulphur dioxide and nitrogen oxide. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth. The precipitation is not necessarily wet or liquid; the definition includes dust, gasses, rain, snow, fog, and hail. Exhaust fumes and open-air burning release carbon dioxide into the atmosphere. When these gases enter the atmosphere, they mix with rainwater to form acids like carbonic acid, sulphuric acid, and nitric acid. They then form acid rain. Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4.
Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants, factories, and automobiles. A chemical reaction happens when sulfur dioxide and nitrogen oxides mix with water, oxygen, and other chemicals in the air.
Acid rain has many adverse effects. It has many ecological effects, especially on lakes, streams, wetlands, and other aquatic environments. Lakes and rivers cannot sustain aquatic life if acid rain flows into the water. It will also lead to a reduction in crop yields. That combination makes waters toxic to crayfish, clams, fish, and other aquatic animals. There will also be irreparable damage to forests and wildlife. Being corrosive, it can cause extensive damage to buildings. An example of an important building that has been corroded by acid rain is the Taj Mahal of India. Acid rain cal also affects human beings. Skin problems such as rashes and itchiness, hair loss, and respiratory problems have been linked to acid rain. Heart and lung problems can also be aggravated by it.
Acid rain affects the biodiversity of the ecosystems. As lakes, streams and other freshwater bodies become more acidic, the number and types of fish and other aquatic plants and animals that live in these waters decrease. As it flows through the soil, acidic rainwater can leach aluminum from soil clay particles and then flow into streams and lakes. The effects of acid rain, combined with other environmental stressors, leave trees and plants less healthy, more vulnerable to cold temperatures, insects, and disease. Acid rain causes poisonous metals to seep into underground drinking water sources, thereby making it unfit for human consumption.
Acid rain affects nearly everything. When acid rain falls in forest areas, it releases toxic metals such as lead and zinc which cause the stunted growth of trees and plants. Acid rain can also change the composition of soil and bodies of water, making them uninhabitable for local animals and plants. Plants, soil, trees, buildings, and even statues can be transformed by precipitation. In this way, acid rain brings about slower growth and the ultimate death of forests.
There are several solutions to stopping manmade acid rain. Scientists agree that the burning of fossil fuels such as oil and natural gas is a major cause of acid rain. This can be done by restricting the use of fossil fuels and focusing on more sustainable energy sources such as solar and wind power. Thus, industries that use fossil fuels such as the automobile, paper, and chemical industries should reduce emissions of harmful gases, which will in turn reduce the acidity of rain.