Background
Square Pharmaceuticals Ltd. (SPL), the pharmaceutical giant in the country, is a trusted name in the industry of manufacturing quality medicines for more than four decade.
SQUARE today symbolizes a name – a state of mind. From the inception in 1958, it has today burgeoned into one of the top line conglomerates in Bangladesh. SQUARE Pharmaceuticals Ltd., the flagship company, is holding the strong leadership position in the pharmaceutical industry of Bangladesh since 1985 and is now on its way to becoming a high performance global player.
| SPL was |
FIRST to manufacture and market Metronidazole, Ampicillin and Cotrimoxazole after the expiry of patents.
FIRST to export antibiotics and other ethical drugs overseas.
FIRST to develop sustained release technology locally.
FIRST to achieve an all time industry high record sales turnover of US$ 25 million.
FIRST to locally produce high tech Metered dose Inhalation (MDI) formulations.
FIRST to locally produce diclofenac sodium in the chemical division.
In addition to these FIRSTs, SQUARE Pharmaceuticals Limited (SPL) was always ahead in introducing new products in the market.
1.1 Profile of Square Pharmaceuticals Ltd.
Corporate Headquarters : SQUARE CENTRE
48, Mohakhali Commercial Area
Dhaka 1212, Bangladesh
Factory : Shalgaria, PabnaTown, Pabna
Established : 1958
Constitution : Public Limited Company
Chairman : Mr. Samson H. Chowdhury
Managing Director : Mr. Tapan Chowdhury
Details of Business : Pharmaceutical Products/ Bulk Chemicals/ AgroVet Products
Authorized Capital : Tk. 1000 million
Paid-up Capital : Tk. 496.8 million
No. of Employees : 2703
ProductRange : Pharma – 91 products in 185 dosage forms
Chemical Division – 7 products
AgroVet Division – 7 products
Name of the Banker : Janata Bank, 1, Dilkusha, Dhaka
CitiBank N.A., 122-124, Motijheel, Dhaka
Standard Chartered Bank, 18-20, Motijheel, Dhaka
Credit Agricole Indosuez, 47, Motijheel, Dhaka
Bank Asia Ltd., 113-116,Tejgaon, Dhaka
Manufacturing Units : 1. Pharmaceutical Division
2. AgroVet Division
3. Chemical Division
Square Pharmaceuticals Ltd. (SPL), the pharmaceutical giant in the country, is a trusted name in the industry of manufacturing quality medicines for more than four decade.
With a capital of Tk. 55,000, a floor space of 3000 sft, and a team of 12 persons, SPL made its humble debut as a partnership firm in 1958.
Within a span of only six years, under the farsighted vision of the management, and the dedicated efforts of the company, its turnover reached the mark of Taka one million. With the growth of turnover came the increase in number of employees, which in 1964stood to 50. At this point of time the partnership firm was transformed into Private Limited Company. In 1964, PL’s Authorized Capital was Tk. 5,00,000 and the Paid up Capital was 4,00,000.
During the mid-seventies (1975) SPL entered into a technical collaboration agreement with Janssen Pharmaceuticals, Belgium, which is a subsidiary of Johnson and Johnson International, USA. Since its inception SPL practices Good Manufacturing Practices (GMP), as recommended by the World Health Organization (WHO).
Another technical collaboration came under way, this time with F Hoffmann-La Roche Ltd., Switzerland, in 1982. But the fruits of this agreement could not be reaped on the account of the Drug Ordinance of 1982. Nevertheless, SPL’s growth was not being stunted. In 1982, turnover reached over Tk. 240 million, and the payroll increased 400 heads, and by 1988 SPL’s turnover exceeded half a billion taka., and the number of employees to 750.
A new factory built in 1987, with all the modern machinery, extensive development of the domestic market, and infiltration into foreign ones like UK and Singapore. Led to this phenomenal growth SPL is the first company in the country ever to export pharmaceuticals finished goods abroad. At present SPL is exporting its products to Nepal, Myanmar, Pakistan, Sri-Lanka, Combodia, and Russia.
With its finished goods already dominating markets at home and abroad, SPL started production of pharmaceutical raw materials groom its new unit, christened as Square Chemical Division, in 1995. The items in production under this unit are Diclofennac Sodium, Amoxycillin, Cloxacillin and Paracetamol.
To sustain the changing environment SPL made convert itself into Public Limited Company in 1991. With a brilliant track record SPL became the first company in Bangladesh to cross the Billion Taka mark turnover in 1992. In 1994 SPL got its share listed in the Dhaka and Chittagong Stock Exchanges. Authorized Capital towered to billion taka and paid up capital by now is taka 250 million. Presently Square is family of 1321 members.
In 1996 an agreement was signed with M/s Tanvec, UK for the establishment of the second formulation unit (Pharma II) at Kaliakoir, Gazipur. This factory, which built with a view to get the approval of USFDA/MCA, is completed in the year 2000, and without doubt, help SPL continue to command its leadership through the next millennium as well.
1.2 Chronology since inception
Year Events
1958 : | Debut of Square Pharma as a Partnership Firm. |
1964 : | Converted into a Private Limited Company. |
1974 : | Technical Collaboration with Janssen Pharmaceutica, Belgium, a subsidiary of Johnson and Johnson International, USA. |
1982 : | Licensing Agreement signed with F. Hoffmann-La Roche Ltd., Switzerland. |
1985 : | Achieved first position in the Pharmaceutical Market of Bangladesh among all national and multinational companies. |
1987 : | Pioneer in pharmaceutical export from Bangladesh. |
1991 : | Converted in to a Public Limited Company |
1994 : | Initial Public Offering of Square Pharmaceutical Shares. |
1995 : | Chemical Division of Square Pharmaceuticals Ltd. starts production of pharmaceutical bulk products (API). |
1997 : | Won the National Export trophy for exporting pharmaceuticals. |
1998 : | Agro-chemicals & Veterinary Products Division of Square Pharma starts its operation. |
2001 : | US FDA/UK MCA standard new Pharmaceutical factory goes into operation built under the supervision of Bovis Lend Lease, UK. |
2004 : | Signing of agreement with ROVIPHARM, Vietnam to manufacture and market SQUARE products under license in Vietnam. |
| Secured the top position for the best published accounts and report for 2003 in the manufacturing category for transparency and excellence in corporate reporting. |
2005 | New State-of- the-Art Square Cephlosporins Ltd. goes into operation; built under the supervision of TELSTAR S.A. of Spain as per US FDA/ UK MHRA requirements. |
1.3 Global Operations of SPL
SQUARE has extended its range of services towards the highway of global market. It pioneered exports of medicines from Bangladesh in 1987. Through its extended marketing operations, SQUARE is now selling its finished goods in many countries of Asia and Europe including:
- Cambodia
- Myanmar
- Nepal
- Pakistan
- ØRussia
- ØSri Lanka
- ØUkraine
- ØYemen
In addition, registration of many of SQUARE’s finished goods in other countries of Asia, Europe, and Africa is now under process.
1.3.1 Partnership and agreement with different world players
1974 : Technical Collaboration with Janssen Pharmaceutical, Belgium, a subsidiary of Johnson & Johnson International, USA
1982 : Agreement signed with F. Hoffmann-La Roche Ltd., Switzerland
1999 : Agreement with Bayer AG, Germany
1999 : Agreement with Eisai Co. Ltd., Japan
1999 : Technical know-how transfer to foreign pharmaceutical company
2000 : Establishment of Square Spinnings Limited.
2001 : Operation of new factory with a view to get USFDA/MCA approval and production support for expanding export operations.
2002 : Establishment of Square – Fashions Limited and Square – Knit fabrics Limited.
2003 : Turnover crosses Four Billion Taka mark.
Overview of Square Pharmaceuticals Ltd.
Chapter 2 |
2.1 Manufacturing Facilities
SQUARE is committed to ensure strict compliance with CGMP norms and regulatory requirements in every phase of manufacturing, quality assurance, and distribution of medicines. To comply with CGMP SQUARE has state-of-the-art technology in production and quality control. In addition, USFDA/MCA standard new plant is now at the completion stage.
Documented Quality Management System (QMS) is integral part of all of SPL operations. People at all levels are committed to adopt advanced technology for continuous development. Being confident with the sophisticated manufacturing and quality assurance technology of SQUARE, multinationals from industrialized countries now have agreements with Square for having their products manufactured in Bangladesh.
2.2 Product Mix
SQUARE has latest technologies for production of a wide varieties of dosage forms including Tablet, Sustained Release formulation, Capsule, Metered Dose Inhaler (MDI), Injectable, Syrup (liquid and dry), Suspension (liquid and dry), Pediatric Drops, Nasal & Ophthalmic formulations, Topical Gel/Ointment/Cream, and oral care formulations.
Current product mix of Square Pharmaceuticals Ltd. comprised of products from the following types of Drug Delivery Systems:
- Tablets
- Non-coated (Vaginal, Dispersible, Chewable, Plain)
- Coated (Sugar coated, Film coated, Enteric coated)
- Sustained released (coated/non-coated)
- Capsules
- Injectables
- Vials containing dry powder for injections
- Small volume parenterals
- Liquids
- Oral (suspension, syrup, drops, and stomatologicals)
- Topical solutions
- Nasal drops
- Dry powders
- Oral (for reconstitution to make suspension, syrup and drops)
- Topical
- Semisolids (creams, ointments and gels)
- Solid suppository formulations
- Metered Dose Inhalers (MDIs)
- Dry Powder Inhalers (DPIs)
- Sterile Ophthalmic Formulations (drops)
Therapeutic Range (Formulation)
| Analgesics/AntipyreticsAnthelmintics Antiallergics Antidepressants Antidiabetics Antidiarrheals Antiemetics/Gastroprokinetics Antifungal Systemic Antigout Antihistaminics Antihypertensive / Antianginal Antiinfectives/Antibacterial Antimalarial Antiprotozoals Antiseptics/Disinfectants Antispasmodics Antiulcerants | AntiviralsBone resorption preparations Dermatologicals Enzymes Expectorants/Antitussives Hematinics Lipid modifiers/Antiobesity Nootropics NSAIDs Ophthalmic preparations Sedatives/Tranquilizers/Muscle Relaxants Bronchodilators/Antiasthmatic Vitamin and mineral preparations Androgen suppressants Anti-migraine Laxatives Neurological |
2.3 Quality
SQUARE is committed to ensure better life through quality medicine. SPL has defined its Quality Policy to fulfill this commitment. To achieve and maintain a steady quality, a range of sophisticated state-of-the-art technology is engaged in operation. SQUARE has adopted the latest quality philosophy by organizing a well-equipped Quality Assurance Department in the plant in addition to Quality Control Department. Above all highly qualified, well-trained, experienced and dedicated professionals are most valuable assets of Square Pharmaceuticals Ltd.
2.4 Research and Development
SPL’s Research and Development is devoted to improve the health care facility of people. Square Pharmaceuticals Ltd. has brought in advanced technology for its Research and Development works. Research & Development includes the bibliographic search aided by a resourceful library, design and selection of process that maximizes efficiency and minimizes the environmental impact, accelerated and long term stability testing, product quality optimization and translation of new scientific insights into the products. R&D Department is also devoted to extensive research and development work in synthesizing bulk chemicals for Chemicals Division. Having started as an importer of technology, R&D Dept. from 1999 has started to export technology to SQUARE’s global customers. To support Research and Development work latest Information Technology (IT) is available with us and SQUARE is now fully prepared to meet the challenge of twenty-first century.
2.5 Chemicals Division for Bulk Drug Manufacturing
In 1995, SQUARE has established a separate division for the manufacturing of bulk drugs. Currently this division is producing the following bulk chemicals for the domestic pharmaceutical companies:
Paracetamol BP/USP
Diclofenac sodium BP
Diclofenac free acid INN
Diclofenac diethylamine
Diclofenac potassium INN
Flucloxacillin sodium BP
Amoxicillin trihydrate BP/USP (compacted and micronized)
Cloxacillin sodium BP/USP (compacted and micronized)
Ampicillin trihydrate BP/USP (compacted and micronized)
Cephalexin monohydrate BP/USP (compacted and micronized)
2.6 Distribution Network
SQUARE is committed to ensure better life through quality medicine. The ultimate motto is to ensure customer satisfaction by exceeding their level of expectations. SPL has 14 Sales and Distribution offices in the following places in Bangladesh:
Depot | Address | Phone |
| Dhaka | 355-356, Tejgaon Industrial Area, Dhaka-1208. | (880-2) 8828775 |
| Pabna | Hospital Road, Salgaria, Pabna. | (880-731) 66580 |
| Bogra | 877/A, MS Road, Bakshi Bazar, Malati Nagar, Bogra | (880-051) 64747 |
| Rangpur | House # 36, Road # 2, R K Road, Islambag , Rangpur. | (880-0521) 63588 |
| Khulna | Alhamdulillah, 25 Usufe Road, Mirzapur, Khulna-9100 | (880-041) 732330, 724654 |
| Barisal | 502/532 South Alekanda, 1 No. C & B Pool, C & B Road, Barisal. | (880-0531) 53661, 56778 |
| Comilla | 400/363, Shishu Mangol Road, Kandirper Comilla. | (880-081) 72320 |
| Mymensingh | 5/A/04 & 5/A/05, Shaheb Quarter, Kachijuli, Mymensingh | (880-091) 55143 |
| Chittagong | House No-1/C, Baijid Bostami Road, East Nasirabad, Chittagong-4000. | (880-031) 654423 |
| Maizdee | 234/B, Hospital Road, Maizdee Court, Noakhali. | (880-0321) 61683 |
| Sylhet | Sabina Mohal, 44, Payra, Darshandewry, Dorga Mohalla, Sylhet. | (880-0821) 725298 |
| Tangail | Biswas Betka, Dhaka Road, Tangail | (880-0921) 53488 |
| Rajshahi | 106, Ambagan, Senanibas Sarak, Rajshahi | (880-0721) 760877 |
| Faridpur | Mission Road, Christian Mission, Police Line, Faridpur | (880- 0631) 63561 |
The extensive marketing network comprising of latest technical and logistic support along with 887 skilled and qualified field staffs is a key to succeed in achieving customer satisfaction level beyond their expectation. The modern warehousing and completely computerized invoicing facilities of SQUARE ensures just-in-time delivery and high customers’ satisfaction.
Resources
| ||||||
| ||||||
| ||||||
|
2.7 New Plant of SPL
SQUARE is now on it’s way to becoming a high performance global company. To this end SQUARE has built a new plant that went into operations by the end of 2001. This plant is the first of its kind in Bangladesh with its MCA of UK and USFDA standard manufacturing and quality assurance facilities.
The implementation of Pharma Unit at Dhaka (Kaliakoir) is carried out under the supervision of M/s. Tanvec Ltd. of UK. In view of the complex and complicated design and high quality standard required for USFDA/EU/MCA specifications the progress of implementation has to be slower. It may be noted that during the year under review total investment increased by Tk. 463,667,368 from Tk. 317,321,566 as on 31-03-98 to 780,988,934 as on 31-03-99 on various heads as shown below:
Square Dhaka Unit Project work was started from October 1996. After technical review and in the best interest of the project in order to achieve quality work as State-of-the-art facility to meet the requirement of the USFDA/MCA Good Manufacturing Practice the original project completion schedule had to be extended.
The Dhaka Unit New Pharmaceuticals Production Facility at Kaliakoir is one of its kind in the country to face the challenge of free world market in the beginning of new millennium.
The State-of-Art Facility incorporated certain basic requirement in the area of Purified Water System. Floor Construction, HVAC System Process Equipment and Validation/ Qualification Documentation as stipulated by USA, EU and other developed countries.
2.8 Export market
In an effort to expand the market for the pharmaceutical products beyond the border, the company has been successful in exporting to several countries at an increasing ratio of its turnover as indicated below:
Taka in ‘000)
| Year | Turnover (Gross) | Exports | % age of GT |
| 2001-2002 | 2,422,785 | 44,361 | 1.83% |
| 2000-2001 | 2,055,418 | 15,082 | 0.73% |
| 1999-2000 | 1,827,983 | 11,503 | 0.63% |
| 1998-1999 | 1,595,590 | 163 | 0.01% |
The company has so far been able to enter into the markets of Russia, Pakistan, Sri Lanka, Myanmar, Nepal, and Cambodia. It is expected that after the Dhaka Unit goes into production the company will succeed in entering the markets of developed countries with expanded share of exports.
2.9 Corporate Financial Highlights
Head Amount
Authorized Capital Tk. 1000 Million
Issued, Subscribed and Paid-up Capital Tk. 496.8 Million
Reserve/Surplus (Retained Earnings) Tk. 872 Million
Share Premium Tk. 800 Million
Long Term Loan Tk. 210 Million
2.10Marketing Performance
2.10.1 Product
Square’s product is viewed among the most quality products in the country. This quality image has increased its credibility among the doctors. It is also pioneer in introducing many new products sought by the doctors. Introducing new products is one of the important objectives of the company. But there are some complaints regarding packaging of the products. But the company has now concentrated in this area and working hard to bring attractive and good packaging
2.10.2 Pricing
Government fixes Price of most of the essential drugs. The number is 118 products. The company can fix price of other products but needs to take approval of government. In pricing a product, Square Pharmaceuticals Ltd. usually follows target pricing. Premium prices cannot be charged, as all the competitor products are similar and not much distinguishable from each other. But prices of some products are still higher than the competitors. But since Square Pharmaceuticals Ltd. does not compromise with the quality, sometimes they have to charge higher to ensure the highest quality possible.
2.10.3 Promotion
Personal selling is the main weapon in pharmaceutical industry. Medical representatives of the company go to the doctors to promote the products. The quality of medical reps is assumed to be the best in Square Pharmaceuticals Ltd. They are selected after a careful scrutiny and are sent to market after some extensive training. This helps Square Pharmaceuticals Ltd. to maintain the quality of its medical reps. Advertising can be given only in magazines related to health profession. Square also utilizes every opportunity to explore this area
2.10.4 Distribution
Square distributes its products all over the country using its own distribution channel. It has a large number of vehicles and sales depots to ensure coverage of the whole country. Its coverage is the best in the country.
|
Corporate Profile of SQUARE
Chapter 3 |
Although Square started its operation in pharmaceutical sector and leader in the field, it is today a synonym of quality toiletries, health products, textile products and AgroVet products. It has also expanded its business in real estate, engineering construction, hospitals, electronic media and other trade & services. SQUARE is now one of the fastest growing and fastest diversifying conglomerate in Bangladesh.
3.1 Square Family
Square family is currently comprised of following companies:
| Square Pharmaceuticals Ltd. | |
| Square Textiles Ltd. | |
| Square Spinnings Ltd. | |
| Square Toiletries Ltd. | |
| Square Consumer Products Ltd. | |
| Square Informatix Ltd. | |
| Square Health Products Ltd. | |
| Square Agro Ltd. | |
| Mediacom Ltd. |
3.2 Associated Companies
As part of vast diversification, SQUARE has the following associated companies:
| Sheltech | |
| Pioneer Insurance Company Ltd. | |
| Mutual Trust Bank Ltd. | |
| National Housing Finance And Investment Ltd. | |
| Aegis Services Ltd. | |
| Maasranga Productions |
3.3 Corporate Headquarters:
Corporate Headquarters of SQUARE is now located at the following address:
SQUARE CENTRE’
48, Mohakhali Commercial Area, Dhaka-1212, Bangladesh
Tel. : (880)-2-8827729 (10 lines); Fax : (880)-2-8828608, 8828609
Email: square@bangla.net, Web: http://www.square-bd.com
3.4 Board of Directors
| Mr. Samson H. Chowdhury | Chairman |
| Mr. Tapan Chowdhury | Managing Director |
| Dr. Kazi Harunar Rashid | Director |
| Mr. Samuel S. Chowdhury | Director |
| Mr. Anjan Chowdhury | Director |
| Mr. Kazi Iqbal Harun | Director |
| Mrs. Jahanara Chowdhury | Director |
3.5 Human resources
In its efforts for human resources development in all spheres of its activities the company offered various courses of training. The company conducted in-house courses of different duration for upgrading skill of 395 employees during the financial year of 1998-1999. 22 employees were sent to various local institutes for training on different topics, 7 employees were sent abroad for training/attending seminars/symposiums on various subjects including general management, 3 expatriates were also invited for holding various discussion forums for officers/staff of the company.
The Breakdown of HR
Job Location | Manager and above | Executive | Non Executive | Total |
Corporate Headquarters | 46 | 123 | 123 | 292 |
Chemicals Division | 2 | 12 | 64 | 78 |
Pabna Plant | 19 | 59 | 459 | 537 |
Dhaka Unit | 14 | 61 | 168 | 243 |
Distribution | 3 | 17 | 174 | 194 |
Field Force(SPL) | 942 | |||
Field Force(Agrovet) | 70 | |||
Field Force(Pesticide) | 25 | |||
Total HR | 84 | 272 | 988 | 2381 |
The Supervision Ratio
Criteria | Manager | Executive | Span of Supervision |
Supervision | 84 | 272 | 1 : 3 |
Executive | Non Executive | ||
272 | 988 | 1 : 4 |
3.6 Activities of Key Functional Departments in SPL
As per the latest organogram SPL had 20 (twenty) functional departments for its operation. The names of the departments are as follows:
- Information Technology (IT) Department
- Medical Services Department (MSD)
- Product Management Department (PMD)
- Sales Department
- Distribution Department
- International Marketing Department
- Market Research and Planning Cell
- Quality Management and Audit Dept.
- Production Planning and Inventory Cell
- Engineering Department
- Production Department
- Commercial Department
- Personnel and Administration Department
- Technical Services Department
- Human Resource Training and Development Department
- Quality Control Department
- Quality Assurance Department
- AgroVet Department
- Accounts and Finance Department
- Accounts (New Venture) Department
Organizational Structure
|
|
|
|
| |||||||||
The following departments manage domestic marketing operation:
- Product Management Department (PMD)
- Sales Department
- Medical Services Department (MSD)
- Distribution Department
- Market Research and Planning Cell
The following paragraphs describe the key functions of the above-mentioned Departments:
Information Technology (IT) Department
The main functions of IT Department are as follows:
- Provide computer and other related accessories supports to all the user
- Maintenance of server and ensure smooth LAN operation
- Provide up to date technical and software support to all the sectors of SQUARE
- Development and maintenance of centralized databases and provide routine and ad hoc reports for management decision making.
Medical Services Department (MSD)
The main functions of MSD are as follows:
Arranging clinical meeting with the physicians on different products
Provide answers to different queries of the physicians through mail or telephone
Arranging education programs for the rural medical practitioners
Publishing of medical journals
Arranging of special promotional campaign of different products
Product Management Department (PMD)
PMD is the core and centralized department for managing the total marketing effort. Basically PMD performs the all planning, implementation of plans as part of marketing management functions. The two key functions of PMD are as follows:
- Introduction of new product into the market
- Manage the existing portfolio to achieve the marketing objectives.
To this end PMD undertakes all relevant activities including the following:
- Preparation of Marketing Plan.
- Designing and development of promotional materials
- Training of field forces
- Feasibility study of new products
- Management of regulatory affairs with Drugs Administration
- Preparation of printed promotional material (literature/pad/brochure/show card etc.).
- All relevant coordination works with supplier/factory and procurement department concerning machinery and raw materials that will be used to manufacture the concerned brands.
Sales Department
- Pay regular visit to the doctors; show the benefits of new existing products with the help of promotional tools.
- Monitor the competitors’ activities.
- Handles initial product queries from doctors and product complain from the market.
- Receive sales order from the retailers /drug stores.
- Coordination among different markets
- Market rearrangement
- Handling different problems of field forces
Distribution Department
Ensure smooth distribution of products to all over the country
Collection of payments from the customers
Performs functions as the representative of SPL at the depot level.
Maintenance of vehicles and depots
International Marketing Department
Exploration of new markets all over the world
Operating of export business in the different countries
Provide training to field forces in overseas countries
Provide all types of documents for registration of SPL’s products in overseas countries.
Market Research and Planning Cell
Performing market survey on the Bangladesh Pharmaceutical Market
Regular prescription share analysis and report generation for SPL market share analysis.
Performing different market research work on different issues
Provide all kinds of support to Field Colleagues in effective planning in the market level.
Quality Management and Audit
Ensure the practices of Quality Management System (QMS) at every stage of operations of SPL in full compliance with ISO 9001.
To monitor the activities to ensure compliance with defined quality policy at every stage of business.
Develop process and instructions for continuous development of operations to increase productivity.
Production Planning and Inventory Cell (PPIC)
Prepare the monthly production schedule of different products
Maintain the inventory status of different raw materials and packaging materials
Technical Services Department (TSD)
- Provide technical support to QC, QA, Production and other departments with regards to any kind of technical issues.
- To procure new Raw Material and production and quality facilities in coordination with Commercial Dept.
- Development of formulation of new products
- Selection of machinery and equipment including spare parts.
- Coordination and follow-up of the designing and implementation of the Master Plan of the factory. All matters related to development of factory facilities.
- Handling various forms of product complaints from market, field forces, and different departments.
- Recipe development and necessary changes in formulations, product improvement.
Current performance of SPL
Chapter 4 |
4.1 Corporate financial highlights
As on March 31, 2006 the corporate financial position of SQUARE was as follows:
| 1. Authorized Capital | Tk. | 1000 Million |
| 2. Issued, Subscribed and Paid up Capital | Tk. | 436.8Million |
| 3. Reserve/Surplus (Retained Earnings) | Tk. | 872 Million |
| 4. Share Premium | Tk. | 800 Million |
| 5. Long Term Loans | Tk. | 210 Million |
| 6. Number of Shareholders (2006) | 13,206 |
4.2 Corporate operational results
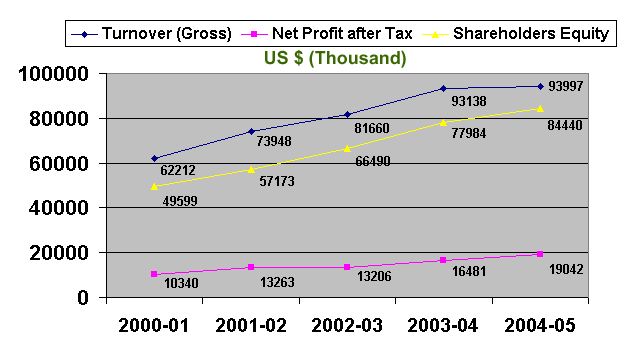
The following three years financial data proves that SPL is operating through maximizing the shareholders’ benefits.
(Figures in thousands)
2003-2004 (12 months) | 2004-2005 (12 months) | 2005-2006 (12 months) | ||
1 | Turnover (Gross) | 3451523 | 4234244 | 4729743 |
2 | Value Added Tax | 450577 | 563433 | 663892 |
3 | Turnover (Net) | 3000946 | 3670811 | 4065851 |
4 | Gross Profit | 1081340 | 1435655 | 1466282 |
5 | Net Profit before Tax | 691636 | 905736 | 929604 |
6 | Net Profit after Tax | 573677 | 759448 | 764885 |
7 | Paid-up Capital | 300,000 | 300,000 | 300,000 |
8 | Earning per Share | 229.47 | 253.15 | 254.96 |
9 | Dividend per Share | 70.00 | 75.00 | 70.00 |
4.3 Output/Capacity utilization
The actual production of the various categories of products (except inhalers) including chemicals increased substantially leading to higher capacity utilization as summarized below:
Product Categories | Units | Production (‘000) | % age increase / (Decrease) | Capital Utilization | |||
2005-2006 | 2004-2005 | 2005-2006 | 2004-2005 | ||||
1 | Tablets | Pcs | 1829089 | 1736561 | 5% | 105% | 231% |
2 | Capsules | Pcs | 295234 | 286350 | 3% | 96% | 119% |
3 | Liquids | Bottles | 21210 | 16105 | 32% | 118% | 153% |
4 | Injectables | Pcs | 13325 | 23075 | (42%) | 74% | 90% |
5 | ENT Preparations | Phials | 8651 | 10778 | (20%) | 192% | 207% |
6 | Opthal Preparations | Phials | 1083 | 966 | 12% | 181% | 116% |
7 | Dry Syrup | Bottles | 4048 | 2634 | 54% | 169% | 88% |
8 | Inhalers | Can | 298 | 148 | 101% | 50% | – |
9 | Basic Chemicals | Kg | 219 | 201 | 9% | 64% | 59% |
10 | Tablets-AgroVet | Pcs | 1262 | 1697 | (26%) | 67% | 99% |
11 | Powder-AgroVet | Gm | 34911 | 15252 | 129% | 189% | 127% |
The capacity utilization rate in some areas of production decreased due to strategic changes in favor of increased production of higher value added products in each category resulting in higher turnover in value.
4.4 Dhaka Expansion
Despite numerous oddities and complex artifices usually associated with a state-of-art plant, the management is in the final phase of implementation of the project with the planning, supervisory and technical support form the consultants e.g. M/s Bovis Lendles and Tanvec of UK and KUPPA collaboration of Thailand, though the target of commissioning had to be revise / shifted a few times in the past. It may be appreciated that the project in hand is only of its kind in the region with USFDA and EU pharmaceutical manufacturing standards requiring highly rigid and unquestionable quality of materials, workmanship, and performance. The process of implementation has often suffered from various hazards of communication, transports, movements and planned accomplishments was not achieved fully due to malfunctioning of ports, airports, airlines, shipping services, etc. Due to carious reasons as are rampant in the country since the project was taken in hand. Considering all these factors and the technical complexities of such a project as well as non-availability of requir5ed man-machine-material in Bangladesh, the delay in implementation of the project would not be termed unusual and abnormal. It may also be mentioned that the consultants also had often failed to identify and consider every possible situation in Bangladesh. In spite of these the unit is scheduled to start functioning by end 2001.
During the year 2005-2006, an amount of Tk. 807,016,104 has been invested on various heads as detailed below:
| Particulars | As on 31-03-05 | As on 31-03-06 | Increase | |
| 1 | Land & Land Development | 86,725,346 | 107,550,519 | 20,825,173 |
| 2 | Building/Civil Work | 318,783,360 | 812,111,406 | 493,328,043 |
| 3 | Imported Plant & Machinery | 348,897,963 | 576,923,068 | 228,025,105 |
| 4 | Other Assets | 6,315,634 | 19,954,395 | 13,638,761 |
| 5 | Interest during Construction Period | 5,577,622 | 47,501,489 | 41,923,867 |
| 6 | Unallocated Expenditures | 14,689,009 | 23,964,161 | 9,275,152 |
| Total Tk. | 780,988,934 | 1,588,005,038 | 807,016,104 |
The remaining implementation work is expected to be now completed by end of 2004 at an estimated final cost of Tk. 1,830 million and commence production thereafter.
Square Dhaka Unit Project work was started from October 1996, which completed by end of March 2000. After technical review and in the best interest of the project in order to achieve quality work as State-of-the-art facility to meet the requirement of the USFDA/MCA Good Manufacturing Practice the original project completion schedule had to be extended.
The Dhaka Unit New Pharmaceuticals Production Facility at Kaliakoir is going to be one of its kind in the country to face the challenge of free world market in the beginning of new millennium.
The State-of-Art Facility incorporated certain basic requirement in the area of Purified Water System. Floor Construction, HVAC System Process Equipment and Validation/ Qualification Documentation as stipulated by USA, EU and other developed countries.
4.5 Export market
The company’s export drive is being continued with great stress. However, there are number of problems in exports of medicines from Bangladesh. As a result, the value of exports in Taka declined by 9.1%. To counter this in future, registration process of products for export purposes has been initiated in few more countries. The company expects to make a break through in exports when the Dhaka Unit is commissioned.
(Taka in ‘000)
| Year | Turnover (Gross) | Exports | % age of gross turnover |
| 2005-2006 | 2,655,952 | 40,324 | 1.52% |
| 2004-2005 | 2,422,785 | 44,361 | 1.83% |
| 2003-2004 | 2,055,418 | 15,082 | 0.73% |
The company has so far been able to enter into the markets of Russia, Pakistan, Sri Lanka, Myanmar, Nepal, Combodia. It is expected that after the Dhaka Unit goes into production the company will succeed in entering the markets of developed countries with expanded share of exports.
4.6 Financial results
The operating financial results of the company for the year under report as compared to the previous year are as follows:
Figure in thousand : BDT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
4.7 Concluding comments
Square Pharmaceuticals Ltd. has a very good working environment where the employees get enough opportunity to show their ability and creativeness. Its communication channel is superb. Informal channel of communication is very active. Many interdepartmental issues are solved in informal meetings, often over a cup of tea. Introduction of LAN has smoothened the process.
Every member of Square Pharmaceuticals Ltd. feels like he or she is a member of the Square family. This family feeling is a big asset for this company. Most of the employees are dedicated and motivated to work. Since good performance is always highlighted and creative ideas welcomed people are eager to contribute their best.
Square Pharmaceuticals Ltd. believes in honesty in every phase of business. The management promotes this idea to its workers. The image of the company is also good all over the country. Customers all over the country recognize Square as a quality company and an honest company.