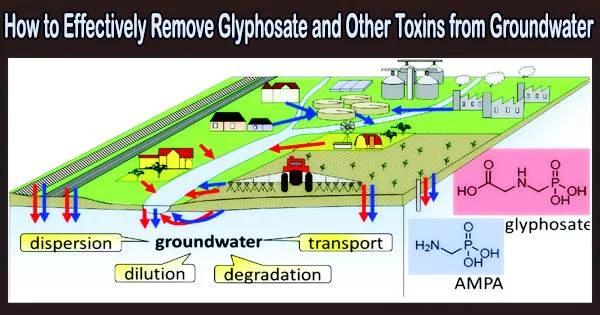
Drinking water contamination is a serious threat to human health. With the technologies now in use, many pollutants, including pesticides, herbicides, hormones, medications, and other chemical substances, cannot be entirely removed from groundwater. The contamination caused by these compounds is also steadily rising.
A recent illustration is the weed killer glyphosate, which is used on a global scale but may be hazardous to both people and the environment.
A team led by Prof. Dominik Eder (TU Wien, Institute of Materials Chemistry) has now developed a new class of materials so-called metal-organic frameworks (MOFs) which can be used to selectively and efficiently remove the herbicide glyphosate from groundwater. The researchers recently published their results in the scientific journal Advanced Functional Materials.
Small volume, large surface
A highly porous, sponge-like network of organic molecules connects small metal oxide clusters to form materials called MOFs. They have an extremely large surface area of up to 7000 m²/g.
“This means that you can fit an entire football field within just one gram of MOFs,” Dominik Eder says. “Consequently, a lot of molecules can adsorb within the pores, making MOFs ideal materials for directly capturing molecules from air and water, such as CO2, inorganic salts and organic pollutants.”
The special thing about MOFs is that they can be customized depending on the application. Shaghayegh Naghdi, lead author of the study, explains:
“Think of MOFs as a large building consisting of individual tiny blocks. Each block is made up of metal atoms or organic molecules and you put them together like a puzzle to achieve the desired functions.”
We selectively burn away a certain part of the organic compound molecules. However, we need to do this very carefully, in order to avoid collapse of the overall micropore structure.
Shaghayegh Naghdi
Mesopores for large molecules
However, a significant obstacle to MOFs’ employment in liquid media is the inaccessibility of the active sites located further inside the substance, where chemical reactions and adsorption processes take place.
The target molecules must diffuse through micropores with sizes of less than 1 nanometer, which is frequently the same size as the molecules themselves, in order to get to these spots. Solvent molecules in liquid media can considerably slow down this diffusion process and block the pores.
To solve this problem, the research group has developed a strategy to incorporate additional pores with a diameter of up to 10 nanometers, so-called mesopores, into the MOFs. How does this work?
“We selectively burn away a certain part of the organic compound molecules,” Naghdi explains. “However, we need to do this very carefully, in order to avoid collapse of the overall micropore structure.”
The team has already tested this strategy for various applications in liquid media.
Successful removal of glyphosate
In collaboration with researchers from the University of Northern British Columbia in Canada, Dominik Eder’s team finally investigated the adsorption of glyphosate from groundwater. Surprisingly, the new material removed three times as much glyphosate in 20% the time as the top adsorbent at the time.
The team also learned that the removal of the organic linkers generates new metal sites through computer simulations performed at the Technion in Israel. These enable the target molecule to diffuse more quickly by forming chemical connections with glyphosate.
“These bonds are strong enough to adsorb glyphosate and similar organic compounds very quickly and efficiently. Yet, they are weak enough to remove glyphosate quantitatively with a simple sodium chloride salt solution, so that these MOFs can be used multiple times,” Dominik Eder explains.