Urea is a critical component found in a variety of products ranging from fertilizers to skincare products. The large-scale production of urea, a naturally occurring byproduct of human urine, is a massive undertaking, accounting for approximately 2% of global energy use and emissions today.
For decades, scientists and engineers have worked to make this process more energy efficient as the demand for fertilizer rises in tandem with population growth. An international research team led by scientists and engineers from The University of Texas at Austin has developed a new method for producing urea that is less harmful to the environment than current methods while producing enough to compete with energy-intensive industrial methods.
Today’s urea production is a two-step thermal process that necessitates high levels of heat and pressure in controlled harsh environments. This new process, on the other hand, requires only one step and is based on an idea known as electrocatalysis, which uses electricity—and potentially sunlight—to trigger chemical reactions in a solution at room temperature in ambient conditions.
Scientists and engineers have devised a new method for making urea that is more environmentally friendly than today’s process and produces enough to be competitive with energy-intensive industrial methods.
“We must reduce emissions all over the world. That is why, instead of this energy-intensive two-step process, we want to develop more sustainable pathways to produce urea via electrocatalysis “Guihua Yu, an associate professor of materials science in the Cockrell School of Engineering’s Walker Department of Mechanical Engineering and co-leader of the team that published a new landmark paper about the process in Nature Sustainability, agreed.
Today, synthetic urea is primarily produced using the Haber-Bosch method, which is regarded as one of the greatest inventions of the twentieth century because it enabled mass fertilizer production and helped increase the global food supply. It combines nitrogen and hydrogen to produce ammonia, which then combines with carbon dioxide to produce urea. This two-step process necessitates heating to 400 or 500 degrees Fahrenheit in order to perform the reaction, consuming massive amounts of energy and emitting significant emissions in the process.
Biofertilizers are a promising tool in agricultural ecosystems because they are a supplementary, renewable, and environmentally friendly source of plant nutrients. They are an important component of the Integrated Plant Nutrient System because they have the ability to convert nutritionally important elements from non-usable to highly assimilable forms while having no negative effects on the natural environment (IPNS).
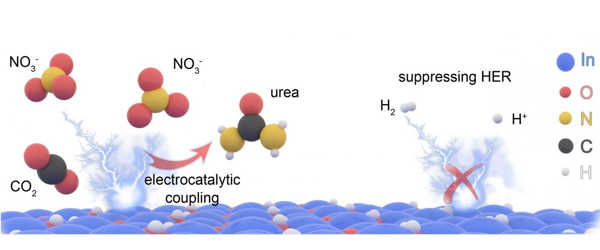
Producing urea through electrocatalysis is a more sustainable and energy-efficient method. However, in the past, this method has not produced enough to make it viable. It produced too many byproducts and required a lot of energy to break the bonds between the molecular building blocks in order to start the reaction.
The primary challenge was identifying the appropriate elements or catalysts to produce an efficient chemical reaction. Instead of nitrogen, the UT team used nitrate to bond with carbon dioxide. Indium hydroxide nanomaterials are used in the catalyst solution.
According to Yu, this highly efficient nanomaterial electrocatalyst has “high selectivity,” which means it produces only what the researchers want it to produce rather than a slew of byproducts. It also produces more urea than previous attempts using electrocatalysis.
“When compared to nitrogen, it takes much less energy to break the bonds of nitrate, which helps produce a much higher yield of urea,” Yu explained.
Yu believes that this formula is applicable to both large and small-scale applications. Individuals could operate electrocatalytic devices and sell them to individual farms, allowing them to produce their own urea for soil. And the hope is to provide alternative solutions to large-scale industrial processes to reduce energy use, which can contribute to a more sustainable future as the population and demand for urea grow.
The next steps in this process will involve further improving yield and selectivity, as well as developing a prototype device capable of scaling up production. In addition, the research team is attempting to find a way to power the process with solar energy rather than direct electricity.