Introduction:
The uniformity of dyed materials mainly depends on the efficiency of fabric preparatory processes. The removal of natural and added impurities thoroughly from the fabrics to achieve uniform whiteness throughout the fabrics is most important for the production concern. Many factors exert significant influences upon the path of technological developments in the wet processing and many of the factors include comparable quality with existing processes with or without value addition, chemical compatibility to provide multi-functional process, cost reduction by minimizing the use of energy and water, increased levels of process control, monitoring and automation, eco-friendly application methods etc. Developments that occur in other branches of engineering and technology are effectively utilized in various processes of textile processing, also, to meet the best requirements for the production sector.
Recent developments in febric preparation process:
Attempts have been made, in the past few decades, to optimize the individual unit operations involved in wet processing to derive the advantages in the respective unit operations1–3. New methods involving low temperature, low pH and eco-friendly chemicals are also explored for reducing the pollution and effluents generated during various operations in industrial sectors. However, a logical approach to conserve energy and materials in the preparatory processes is to shorten the sequence by combining the three operations, desizing, scouring, bleaching, commonly known as DSB process. Most of the combined preparation processes have failed mainly due to inadequate removal of seed coats/mote. Various combined processes have been developed in the past using different chemicals and also using new formulations for scourant 1– 3.
Peracetic Acid (PAA) Bleaching an Eco-friendly Alternative:
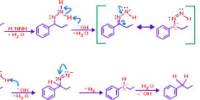
Any substitute to the traditional bleaching agent NaOCl, should be a product with comparable redox potential. In case of low temperature bleaching it has been introduced peracids as stronger oxidizing agents than hydrogen peroxide (Table- 1). Use of PAA as a bleaching agent has been discussed by many authors for different fibres like cotton, flax, nylon 4 –9. The rate of decomposition and consumption of PAA vary over a range of bleaching temperature at different pH with varieties of alkalis. PAA consumption is slow when sodium hydroxide (NaOH) is used as an activator, at different values of pH and temperature, whereas consumption is quicker and rapid with inclusion of magnesium carbonate. PAA is produced industrially by mixing acetic acid with hydrogen peroxide in presence of an acid catalyst. At higher pH and temperature, PAA decomposes spontaneously to produce acetic acid and oxygen. The chemical reaction can be expressed as in mechanism -1.
Fabrics treated with PAA at neutral pH at room temperature for about an hour followed by alkaline peroxide bleaching at 90°C have shown a brightness of greater than 90 Berger units, significantly with less fibre damage and crease marks. PAA is used for the removal of heat setting discoloration from nylon, carried out at pH 6.0 –7.5 for about an hour at 80°C using 0.3% solution. The similar process also could be used for viscose rayon, secondary acetate and triacetate materials.
Table- 1: Potential of oxidizing agents
| Agents | Potential (eV) |
| Ozone | +2.07 |
| PAA | +1.81 |
| ClO2 | +1.57 |
| NaOCl | +1.36 |
The main advantage of bleaching with PAA instead of hydrogen peroxide is that satisfactory degree of whiteness can be obtained at 60°C, within 40 min at neutral pH without addition of auxiliary agents. Optimum whiteness index can be achieved at temperatures around 50°C and 90°C and bleaching time over 60 min yields significantly higher brightness4. For a given degree of whiteness, the percentage strength loss and the extent of chemical damage are the least for PAA followed by hydrogen peroxide10. The absorbency time and tearing strength decreases steadily up to a pH 7 value and the bleached fabrics show comparable fastness properties with reactive dyes5.
Implementation of Single Stage Preparatory Process:
Single stage preparatory process using hydrogen peroxide has been developed successfully for starch and acrylic-base sized textile materials previously 11,12 . In such processes, caustic soda provides required alkalinity for scouring and activation of hydrogen peroxide and when activated, hydrogen peroxide degrades the sizing materials at lower temperature and at higher temperature, bleaching occur along with completion of desizing. Higher alkalinity at elevated temperature produces efficient scouring action. A self-emulsifiable solvent system of bleaching has been developed to combine the three different processes involved in the preparatory process. The system uses a high proportion of water, very low levels of solvents and hydrogen peroxide. The presence of hydrogen peroxide helps both desizing and bleaching and the emulsified solvent results in the scouring of cotton fabric11. Since the system involves very low quantity of solvent content, need for a solvent recovery plant is obviated. In the case of sodium chloride (NaCl)- hydrogen peroxide (H2O2) system, free radical mechanism( Mechanism-2) is responsible for the bleaching action. Various free radicals created during the treatment resulted in disintegration and destruction of foreign matters present in the cotton. The bleaching effect is more distinct with peroxide than sodium chlorite, even at the higher concentrations. Presence of co-oxidants impedes the decomposition of each other, especially at their lower concentrations. The reactions under alkaline medium are initiated as chain reaction by the production of different free radical in different steps. The different step of the reaction is shown below:
The HO· and HOO· radicals react with the chlorite ions and, as the result, a reaction chain is perpetuated as suggested as:
These free radicals enhance the bleaching effect of NaCl by H2O2 when used at their higher concentration. Thus created free radicals seem to disintegrate the impurities and destroy the coloring matters of the cotton. Various recipes that have been developed for the single stage preparatory process summarized in Table -2. In case of the hypochlorite-solvent assisted single stage preparatory process 13-15, the whiteness index and tensile strength exhibit approximately a linear relationship with available chlorine in sodium hypochlorite solution at various treatment at a time range from 45 to 225 min. Better wetting time is obtained at the scouring agent concentration of
8% at a temperature of 50°C – 55°C, which is closer to the cloud point of the non-ionic emulsifier used in the recipe. In the peroxide-alkali process, absence of either sodium hydroxide or hydrogen peroxide in the peroxide based process results very low weight loss, indicating very low efficiency11.
Table- 2: Recommended recipes for single stage preparatory process (SSPP) :
| Process Name | Recipes/Procedure | Ref. |
| Peroxide- alkali media | H2O2: 1.0 g/L, NaOH:10 g/L, Wetting agent: 1.0 g/L, Temperature: 95° C, Time: 120 min, pH = 10.5-11.5( using Soda ash) | 4 |
| Peroxide-solvent assisted scourant- Starch sized fabrics | Scouring agent : 4%, Peroxide: 2%, Sodium silicate : 1%, Wetting agent : 0.1%, Temperature : 95°C, Time :180 min, pH = 10 | 5 |
| Peroxide-solvent assisted scourant – crylic-base size | Scouring agent : 4% , Peroxide :1%, Sodium Silicate : 1%, Wetting agent : 0.1%, Temperature : 95°C, Time :180 min, pH = 10 | 5 |
| Peroxide-solvent assisted scourant | Scouring agent : 4% o.w.f., H2O2: 1%, Na2SiO3 : 2%, Na3PO4 : 2%, or tetra sodium pyrophosphate, pH = 11, Time :12 min – 16 min/24 h at 40°C | 11 |
| Hypochlorite-solvent assisted scourant | Solvent based scouring agent : 2%, NaOCl : 6 g/L (avg. Cl2 ), Temperature : 40°C, Time :180 min, M : L –1: 20, pH = 11 | 8,9 |
| Sodium Chlorite – peroxide process | NaClO2: 3 g/L, Na2HPO4: 10 g/L, NI Wetting agent : 2 g/L, pH =10, Time : 90 min, Temperature : 95°C, M : l – 1 : 20 | 7 |
| Sodium Chlorite –Solvent assisted scourant | Sodium Chlorite : 0.8 – 1.95%, Scouring Agent : 2%, (activators as stated in the text), Temperature : 30 – 55°C, Time : 5 h – 14 h, pH = 4.6 (buffered) & 8 – 9(un-buffered) | 6 |
| Peroxide – alkali process | Sodium Hydroxide : 20 g/L, Hydrogen Peroxide : 30 ml/L -40 ml/L, Peroxidisulphate : 5 g/L, Sodium Silicate : 20 ml/ L, Surfactant : 10 g/L, Stabilizer : 5 ml/L | 5 |
| Flash steam process | Ciba Tinoclarite FS :15 ml/kg – 30 ml/kg, NaOH(100%) : 30 gm/kg – 50 gm/kg, H2O2 (35%) : 49 ml/kg – 90ml/kg | 10 |
In the SSPP, an attempt has also been made to improve the efficiency by carrying out the process using microwave of 600w for duration of 30sec to 3 min. The process involves steaming of the fabric with the simultaneous exposure to the microwave energy. In another narrative apsteam procedure has been used to combine all the preparatory processes into a single step.
Application of Plasma Technology:
The plasma technology is considered to be very interesting future oriented process owing to its environmental acceptability and wide range of applications. Plasma is the fourth state of matter besides solid, liquid and gas which contains ionized gas comprising of ions, electrons, atoms and molecules. Presence of free electrons and other charged particles converts plasma as electrically conducting and responsive to electricity. The plasma modifies the fabric surface by the bombardment with high energy electrons and ions shown in
The structure and construction of yarn play major roles in deciding the efficiency of plasma processing. The presence of impurities in the raw fibres, additives present in the yarn and fabrics also intervene during the plasma processing. The free radicals produced in the treatments can undergo reactions depending upon the gases present in the atmosphere. The acidification of raw cotton increases with increase in purity of cotton fibres and the change in pH was to the extent of about 1.0 for raw cotton whereas it increases to above 2.0 for bleached fibres. Even very high power supply with prolonged treatment results in no significant change in tenacity and elongation at break values for both PET and cotton fabrics.
The voids and cracks developed in PET also aid penetration of moisture. Plasma treatment offers effect which are often decaying in nature, i.e., ageing or durability effect. Grafting of flame retardant monomers has been successfully carried out on the cotton fabrics to obtain durable effects than that is possible with conventional surface deposits. Development of self -cleaning cotton textiles through RF-plasma, MW-plasma and UV -radiation to introduce functional groups to anchor TiO2 on textile surface shows the formation of TiO2 crystallites with 5 – 7 nm size immediately, from the precursor 19. Plasma processing has been attempted in desizing of PVA sized viscose rayon, starch sized cotton yarns, scouring of cotton and wool fibres using cold plasma20. These reactions occurred mainly due to ablation of surface, removal of starch and hydrophobic substances. The subsequent washing reduces the wetting time to less than1 sec.
The percent desizing ratio21 reaches up to 97%, air-oxygen-helium plasma yields better weight loss (up to 2.8%) compared to air-helium plasma 2.3%. The contact angle decreases considerably after oxygen plasma treatment compared to untreated samples and the plasma treatment followed by scouring results in the residual wax content to 8.0% in cotton and wool. Surface modification of textile materials to create a reactive surface suitable for dyeing and finishing treatments has been attempted by many
researchers17, 19,. The effect of plasma treatment on fabrics made of various fibres and the relevant results are summarized in Table-3.
Table- 3: Effect of plasma finishing on various fabrics.
| Fibre | Process Name | Procedure | Effects | Result |
| Cotton | Hydrophilicity and wettability (RF- plasma)
Hydrophobicity | Air – O2 plasma, low pressure 0.6-8 mbar
Air-O2 plasma, low temperature at 9 pa pressure, 70w – 120w CF4 ,C3F6 plasma 100, 300w, 50 m & 75 m, Torr for CF4 50-160w, 50 m – 150 m | Weight loss, increase in C = O, COOH content, increases in vertical wicking Lower results for CF4 than C3F6 | Strong etching
Generates greater changes in fibres C3F6 polymerizes by plasma and plasma inducing methods. CF4 yields plasma polymerization atomic fluorine only. |
| Linen | Wicking (RF- plasma) | Ar – O2 plasma, pressure 15 pa with power 100w, 200w | Weight loss, etching effects, wicking rate decreases | No significant effects on prolonged exposure but causes degradation |
| Flax | Topographical study (RF- plasma) Hydrophilicity | Ar- O2 plasma, 200w, frequency 13.56 MHz, pressure 15pa
Water vapor, power, 100w and pressure 100pa | Etching of surface and revelation of fibrillar structure
Removal of fatty layer, generation of hydrophilic groups | O2 plasma shows faster rate, bigger micropores, shrinkage
Epicuticle is removed |
| Wool | Shrink resistance (Glow discharge) (RF- plasma) | O2-plasma, low temperature, at 10pa pressure | Felting decreases, becomes shrink resistance, alkaline solubility increases and dyes faster | Micropores and cleft created. Subsequent enzyme treatment enhances handle and dyeability |
| Polyester | Increased surface energy and wettability (RF-plasma) | O2-plasma, low pressure 1– 10pa. low temperature, glow discharge | Formation of new groups as – OH, – C=O. Surface energy increased from 24 mJ/m2 to 71 mJ/m2 | Creation of surface roughness, loss of hydrogen, appearance changes due to low reflectance |
| Nylon | Water repellency (RF- plasma) | CF4 plasma power, 100w & 4pa | Absorption of F atoms on surface | Air resistance and glossiness improves |
Generates greater changes in fibres
C3F6 polymerizes by plasma and plasma inducing methods. CF4 yields plasma polymerization atomic fluorine only.
Linen Wicking (RF- plasma) Ar – O2 plasma, pressure 15 pa with power 100w, 200w Weight loss, etching effects, wicking rate decreases No significant effects on prolonged exposure but causes degradation
Flax Topographical study
(RF- plasma)
Hydrophilicity
(RF -plasma) Ar- O2 plasma, 200w, frequency 13.56 MHz, pressure 15pa
Water vapor, power, 100w and pressure 100pa Etching of surface and revelation of fibrillar structure
Removal of fatty layer, generation of hydrophilic groups O2 plasma shows faster rate, bigger micropores, shrinkage
Epicuticle is removed
Wool Shrink resistance (Glow discharge)
(RF- plasma) O2-plasma, low temperature, at 10pa pressure Felting decreases, becomes shrink resistance, alkaline solubility increases and dyes faster Micropores and cleft created. Subsequent enzyme treatment enhances handle and dyeability
Polyester Increased surface energy and wettability (RF-plasma) O2-plasma, low pressure 1– 10pa. low temperature, glow discharge Formation of new groups as – OH, – C=O. Surface energy increased from 24 mJ/m2 to 71 mJ/m2 Creation of surface roughness, loss of hydrogen, appearance changes due to low reflectance
Nylon Water repellency
(RF- plasma) CF4 plasma power, 100w & 4pa Absorption of F atoms on surface Air resistance and glossiness improves
Dyeing in Supercritical Fluids (DSF):
Supercritical fluids are highly compressed gases and combine valuable properties of both liquid and gas21. Supercritical fluids have solvent power similar to a light hydrocarbon for most solutes. Solubility increases with increasing density (i.e., with increasing pressure). However, fluorinated compounds are often more soluble in CO2 than in hydrocarbons, which increased solubility, is important for polymerization. A liquid can be converted to a supercritical fluid by increasing the temperature and consequently its vapor pressure and simultaneously with increasing pressure. A closed system thus reaches the supercritical state, where no boundary between the liquid and gaseous state can be distinguished.
Any increase in pressure subsequently results, increase in dielectric constant and the dissolving power to a greater extent. Carbon dioxide is frequently used as a solvent because of some inherent advantages associated with the system like, non-toxic, non-corrosive and non-hazardous nature; CO2 is produced commercially and can be transported easily. The critical points of the CO2 can be achieved easily compared to other gases. The dissolved dyestuff available for diffusion into the boundary layers in the supercritical fluid is absorbed and diffuses into the fibres. The state of the dyestuff in a super critical solution can virtually be described as gaseous. Supercritical CO2 has almost a plasticizing effect which accelerates the diffusion processes by increasing the chain mobility of the polymeric molecules. This means that it will be absorbed by the fiber at a rate comparable to the high diffusion rates corresponding to that of a
The distribution dyestuff-fluid can be continuously shifted in favor of the polymer until after expansion of the gas to standard pressure the solubility in the fluid will be equal to zero, where a theoretical exhaustion level of 100% is achieved.
Foam Finishing:
The wet processing of textile materials includes highly energy consuming operations, approximately to 80% of total energy requirement of all the operations. Out of this, about 66% of the energy is consumed in heating and evaporation of water from the fibres. Invariably, the liquor retained in the fabric is distributed 22 within and between the fibres in the form of capillary liquid in the available spaces between the yarns and also on the surface of the textile material, i.e., surface bound water. Squeezing the fabric between the nips eliminate the excess liquor available on the surface of the fabric and the interstices of the yarns, which depends on the nip pressure, hardness of rubber, roller diameter and machine speed or fabric speed. The concept of low add-on is based on the controlled transfer of a reduced quantity of liquor from a dipping roller to the fabrics. Moreover foam application is different from the low add-on technique since air is used to dilute the liquor, which is not the case in the earlier ones. In the foam process the liquor is diluted using the air instead of water that is normally used to apply the chemicals over the textile materials.
In foam finishing, most of the water is replaced by air, which leads to a reduction of energy requirements in the drying processes resulting in substantial savings in energy cost. Foam is a colloidal system comprising of mass of gas bubbles dispersed in the liquid continuum23,24. Foam can be generated by mechanical air blowing, through excess agitation or chemically by introduction of foaming agents or combination of these methods. The relative proportions of air and liquid phases in the foam are designated by blow ratio. Foam stability, density and diameters are the important parameters that need constant attention25. A varying bubble size represents an unbalanced bath density. Foam density in general, varies between 0.14 g/cc – 0.07 g/cc for the foam finishing and 0.33 g/cc – 0.20 g/cc in the foam printing. The selection of the density of the foam depends on the fabric weight and needs to be increased with increasing fabric weight. Foam viscosity depends on the foam density and viscosity of the un-foamed liquor. Increase in foam viscosity results increased foam stability. Bubbles with smaller size are more stable than bigger bubble size and the bubble diameter ranges, generally, from 0.001 mm – 2.0 mm depending on the generation systems. Bubbles or foams, inherently, do not thrive in higher energy environment since higher energy levels results in the destability of the foam25. Destabilization of the
foam is also caused by creation of the faults in the foam structure at the air/liquid/air interface. The destruction of foam after application on to the fabrics can be achieved by conventional padding or vacuum application or combination of both.
Foam application technique can be used23,24 in the fabric preparation, dyeing and printing, DP finish, softening, soil-release finish, water, oil repellant finish, FR finish, anti-static finish, mercerization, etc. The foam can be applied either on one or both sides of the fabrics. Horizontal padder, kiss roller coating, knife over the roller coating, knife on air system and slot applicator system are commonly employed in foam applications. Conservation of water and energy has been the centre of research during the past and today also 26,27 and foam finishing process can be used to achieve 80% of water consumption28, the energy consumption by 60% – 65% in the form of gas, electricity depending upon the type of finishing treatment used, obnoxious gases and their related pollution can be minimized29. The chief advantages of foam application techniques with foam finishing treatment reduces the pay back period to as low as six months to two years 24, 25, 9, 10, 11.
Ultrasonic Assisted Textile Processing:
Sound waves have been classified into infra-sound (up to 16Hz), audible sound (16 Hz –16000 Hz) and ultrasound which include sound waves higher than audible sound with a frequency above approximately 16 kHz up to 106 kHz. Unlike gases and liquid, in solids both longitudinal and transverse waves are transmitted. The effects of ultrasonics actually arise from the way in which sound is propagated through the medium. In liquids, longitudinal vibrations of molecules generate compressions and rare factions, i.e., areas of high and low local pressure. The latter results in the formation of cavities, i.e., very small vapor bubbles of 500nm in size, which can collapse and cause shock waves through out the medium. The formation of cavitations depends on the frequency and intensity of waves, temperature and vapor pressure of the liquid 30. Cavitation is the principal physical phenomenon behind all the effects of ultrasound in most of the treatments. Cavitations refer to the formation, growth and collapse of vapor or gas bubbles under the influence of ultrasound. If the bubbles collapse in the vicinity of a solid surface such as a textile material, it results in the formation of a high velocity micro jet with the velocities as high as 100 m/s –150 m/s directed towards the solid surface31. These micro jets can give rise to intra yarn flow, increase in the rate of the mass transfer between the intra-yarn and inter yarn pores. On the other hand, they may be carried along with the sound waves if they do not collapse immediately. This, in turn, pushes water along with the bubbles producing a flow of water called streaming away from the sound source. The two phenomena attributed to ultrasound are the rapid movement of liquids caused by variation of sonic pressure which subjects the solvent to compression and rarefaction and micro streaming. Simultaneous formation and collapsing of tiny air bubbles result in a large increase in pressure and temperature at microscopic level. Heat induced by the ultrasonic process is adequate for dyeing process and thus eliminates the need for external heating in many cases. Advantages of ultrasonics in textile wet processing include energy saving by reduced processing temperature, time, and lower consumptions of auxiliary chemicals and further processing enhancement by overall cost control33. Ultrasonic method has been effectively utilized in various fabric preparation processes including desizing, scouring, bleaching, mercerization and auxiliary processes, like washing and laundering31, 34-36. Desizing of cotton and nylon fabrics under ultrasonic treatment results complete removal of oils used in the size recipe while the treatment without ultrasound shows residual oil stains. Effect of ultrasonic in enzymatic scouring has been tested using both acidic pectinases and alkaline pectinases and found to have increased wettability of all treated samples both tests compared to the conventionally treated samples. Ultrasonic treatments help to reduce the processing and temperature required for a result comparable to the normal bleaching and subsequent dyeing processes in terms of absorbency and fastness properties. Ultrasound is used for mercerizing 100% cotton fabrics in the after treatment and speeds up the process up to 2 – 3 times. Ultrasonic also has been used for evaluating its impact on washing the fabrics and garments under the simulated stain conditions on 100% PES and P/C (65/35) blends using the detergent of 1 g/L. Attempts have been made to analyze the effect of ultrasonic in dyeing processes on almost all types of fibres using direct, reactive, acid and disperse dyes. Ultrasonic waves accelerate the rate of diffusion of the dye inside the fibre with enhanced wetting of fibres. Acoustic irradiation of the liquor results in a higher and more uniform concentration of dyestuff on the fibre surface, making it available for ready diffusion into the fibre interior.
The influence of ultrasonic on the dyeing system has threefold effects namely, dispersion effect, i.e., breaking up of micelles and high molecular weight aggregates into uniform dispersion in the dye bath, degassing by the removal of dissolved or entrapped gas molecules or air from fibre capillaries and interstices at the cross over points of the fabric into liquid thereby facilitating a dye-fibre contact and accelerating the rate of diffusion of the dye inside the fibre by breaking the boundary layers covering the fibre and accelerating the interaction between dye and fibre. These above mechanisms may be in effect individually or in combination of the above. Extracts obtained various natural plants sources like Cassia fistula, Impatiens balsamina, Al root bark, barks of eucalyptus, which grow abundantly in tropical and sub-tropical forests have been obtained to the cellulose fibres and their blends using ultrasonicators for both extraction as well as for the application purposes 37,38. An attempt has been made to compare the dyeability of both PET and PBT in presence of ultrasound using disperse dyes. The effect of carrier and ultrasound together was significantly larger than either individually. Table- 4 shows various frequencies and power output required for various textile wet processing operations including effluent treatment.
Table – 4: Frequency and power output for wet processing.
| Ultrasonic Frequency ( kHz) | Power output (w) | Application | Ref. |
| 30,47 | 230 | Laundering of solid febrics | 3 |
| 20,24 | 600 | Desizing | 4 |
| 20 | ….. | Natural dyes on cotton and blends | 10,12 |
| 26 | 120 | Disperse dye on PET | 15 |
| 20 | 180, 600 | Dyeing with disperse, direct, acid, basic dyes | 1,2 |
| 520 | ……. | Effluent treatment | 16 |
Conclusion:
The development of unique textile wet processing technology comes with a host of challenges. Finishes must be durable during the finishing process; stable in the presence of other chemicals; wash-fast; and evenly and consistently applied. Finishes also need to be financially feasible and environmentally friendly. The typical dyeing process involved the use of chemicals and thermal energy, which can be reduced, by using ultrasound energy. Among the wet processes, application to dyeing seems to be most advantageous, followed by finishing and preparation processes. There may be a possibility of reducing the pollution load on effluent water. Since recently, however, the plasma Technology is being introduced in textile industry as well. The processes the methods are economical and reduce the environmental impacts caused by the chemical textile industry. Dyeing with super critical CO2 is still at its infancy. It has been proved time and again that it’s successful at laboratory scale. Large amount of research input is needed for system integration.