According to a new study, the Cdc42 gene is required for the correct polarity of Sertoli cells that support sperm production in mice. The findings could hasten efforts to develop a non-invasive diagnostic test for male infertility.
Cincinnati Children’s researchers appear to have flipped another piece of the unsolved puzzle of male infertility. A team led by co-first authors Anna Heinrich, BS, Bidur Bhandary, Ph.D., and senior author Tony De Falco, Ph.D., published findings in Cell Reports that shed new light on how sperm production can go wrong when a specific gene fails to function at the right time.
“By learning more about how Cdc42 functions within the male reproductive system, we can use this gene as a potential biomarker for infertility or reduced testicular function,” De Falco says.
Targeting this gene specifically in the testis would be difficult without affecting other organs, cell types, or cellular processes. By learning more about how Cdc42 functions within the male reproductive system, we can use this gene as a potential biomarker for infertility or reduced testicular function.
Tony De Falco
What’s a Sertoli cell?
The research focuses on the function of Sertoli cells, which line up along the inside walls of long narrow tubes in the testes called seminiferous tubules, which are used for sperm production. Sertoli cells, also known as “nurse cells,” serve as docking stations for nutrients to develop sperm cells.
The team discovered that when the gene Cdc42 is missing or not functioning, it disrupts the polarity of Sertoli cells, causing some to be attached or oriented incorrectly inside the seminiferous tubules. The misaligned cells become less capable of supporting sperm cells, and some of the misaligned Sertoli cells die off, reducing the testes’ ability to produce an ongoing supply of sperm.
The researchers also discovered that this sperm production disruption occurs in mature adult testes but not in juvenile testes.
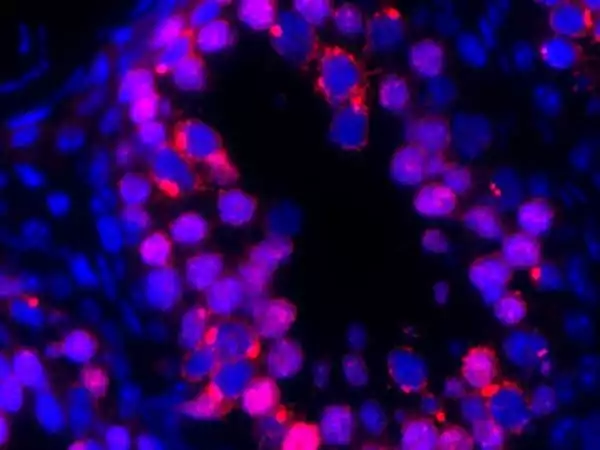
Why is this discovery about Sertoli cells important?
In the United States, nearly one in every seven couples is infertile. Male infertility is a factor in many situations. It is unclear how many cases of infertility can be traced back to malfunctioning Sertoli cells, but treatments that improve Sertoli cell function should improve male sperm production.
Attempting to directly alter the Cdc42 gene is unlikely to be an option, at least in the short term. This is due to the fact that the Cdc42 gene plays numerous roles throughout the body. “Targeting this gene specifically in the testis would be difficult without affecting other organs, cell types, or cellular processes,” De Falco says.
Furthermore, even if a treatment can be limited to Sertoli cells, any genetic variations that result pose a significant risk of being passed down to future generations, potentially resulting in unknown side effects. “For these reasons,” De Falco says, “a high bar must be set for regulating, testing, and validating the use of gene-editing technologies in human patients.”
More likely, these discoveries will aid in the development of improved diagnostic tests to better identify specific causes of male infertility. Currently, diagnosing Sertoli cell malfunction usually necessitates a biopsy. If a non-invasive test reveals that a child is at high risk of developing misaligned Sertoli cells as an adult, the individual may benefit from testicular or sperm biobanking.
Peripheral nerve research provides inspiration
While previous research in reproductive sciences had raised concerns about Cdc42’s role in fertility, the team credits collaboration with the lab of Nancy Ratner, Ph.D., an expert in nerve tumors at Cincinnati Children’s, as a critical step in advancing the work.
“Their lab discovered that Cdc42 is required for peripheral neuron function, most likely by regulating cell polarity,” De Falco explains. “Because Sertoli cells are highly polarized, similar to neurons, we hypothesized that Cdc42 mutations would disrupt their function as well. We were able to use the same mouse model they did because it also deleted Cdc42 in Sertoli cells.”
Next steps
De Falco’s team intends to conduct additional research into cell polarity malfunctions in other types of male fertility cells.
“When germ cells differentiate into sperm, they are also very polarized, with a head-and-tail morphology. It would be interesting to see if Cdc42 regulates cell polarity in sperm cells in the same way that it does in Sertoli cells. We’d also like to see if cell polarity is important for function in other types of testicular cells, such as immune cells, vascular cells, and steroid-producing Leydig cells.”