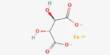
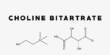
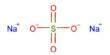
Synthetic biology (SynBio) is a multidisciplinary discipline of study that focuses on living systems and organisms, and it employs engineering concepts to create new biological parts, devices, and systems, as well as modify existing natural systems. It is a science and engineering interdisciplinary topic that focuses on creating and building biological systems or components with innovative functions and capabilities. It integrates biological, engineering, computer science, and chemical principles to develop or change biological organisms or biological pieces for practical applications.
It is a branch of science that encompasses a wide range of methodologies from various disciplines, including biotechnology, biomaterials, material science/engineering, genetic engineering, molecular biology, molecular engineering, systems biology, membrane science, biophysics, chemical and biological engineering, electrical and computer engineering, control engineering, and evolutionary biology.
The primary goals of synthetic biology include:
- Redesigning biological systems: Synthetic biologists study and change biological creatures’ genetic code, cellular processes, and metabolic pathways in order to engineer them for specific tasks or uses. Changing an organism’s DNA, introducing new genes, or reprogramming cellular activities are all examples of this.
- Creating new organisms: It allows for the production of completely novel organisms that do not exist in nature. These creatures, sometimes known as “synthetic organisms” or “synthetic life,” can be programmed to perform specific activities such as the production of biofuels, medicines, or other useful substances.
- Developing bio-based technologies: It has a wide range of applications, including medicine, agriculture, energy, and environmental cleanup. It can, for example, be used to create bacteria that can clean up oil spills or to genetically engineer crops to be more resistant to pests and illnesses.
- Standardizing biological parts: One of the key principles of synthetic biology is the development of standardized biological parts, much like standardized electronic components. These standardized parts, such as genetic sequences, promoters, and regulatory elements, can be used as building blocks for constructing biological systems.
- Ethical and safety considerations: As synthetic biology has the potential to create powerful and potentially risky technologies, there is a strong emphasis on ethical and safety considerations. Researchers and policymakers work to ensure responsible and safe practices in the field.
It entails designing and building biological modules, systems, and devices, as well as re-engineering existing biological systems for practical uses. Furthermore, it is the discipline of research that focuses on engineering new abilities into existing creatures in order to modify them for practical reasons.
In recent years, synthetic biology has made considerable advances, and it holds great potential for addressing a variety of global difficulties, such as producing sustainable energy sources, developing novel medications and cures, and addressing environmental issues. However, it creates ethical, regulatory, and safety problems that must be addressed through thorough oversight and responsible research techniques.