About Enzyme Catalyzed
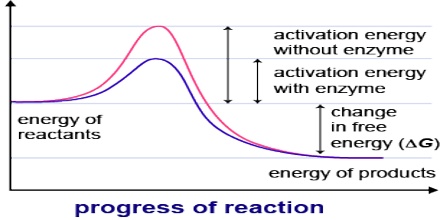
Enzymes are proteins that accelerate biochemical transformations by lowering the activation energy of reactions.
Enzyme catalysis is the increase in the rate of a chemical reaction by the active site of a protein. The protein catalyst (enzyme) may be part of a multi-subunit complex, and/or may transiently or permanently associate with a Cofactor. Catalysis of biochemical reactions in the cell is vital due to the very low reaction rates of the uncatalysed reactions at room temperature and pressure. A key driver of protein evolution is the optimization of such catalytic activities via protein dynamics.
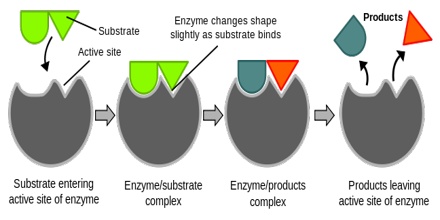
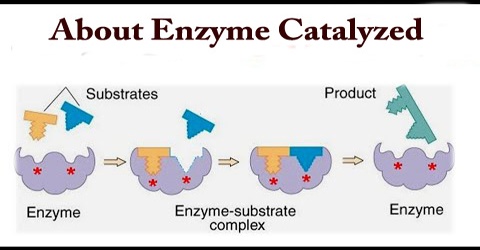
Synthetic catalysts are used to accelerate a variety of industrial processes and are crucial to the chemical manufacturing industry. However, catalysts are also found in nature in the form of enzymes. Enzymes are proteins that are able to lower the activation energy for various biochemical reactions. They do this by binding the reactant(s), known as the substrate(s), to an active site within the enzyme. At the active site, the substrate(s) can form an activated complex at lower energy. Once the reaction completes, the product(s) leaves the active site, so the enzyme is free to catalyze more reactions.
Key Points
- Enzymes are a special class of catalyst that can accelerate biochemical reactions.
- Enzymes are proteins that bind reactants, or substrates, in regions called active sites.
- Upon binding, conformational changes in enzymes result in stabilization of the transition state complex, lowering the activation energy of a reaction.
The mechanism of enzyme catalysis
In order for a reaction to occur, reactant molecules must contain sufficient energy to cross a potential energy barrier, the activation energy. All molecules possess varying amounts of energy depending, for example, on their recent collision history but, generally, only a few have sufficient energy for reaction. The lower the potential energy barrier to reaction, the more reactants have sufficient energy and, hence, the faster the reaction will occur. All catalysts, including enzymes, function by forming a transition state, with the reactants, of lower free energy than would be found in the uncatalysed reaction. Even quite modest reductions in this potential energy barrier may produce large increases in the rate of reaction.
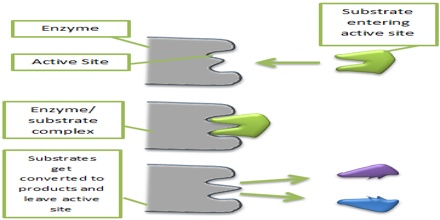
There are a number of mechanisms by which this activation energy decrease may be achieved. The most important of these involves the enzyme initially binding the substrate(s), in the correct orientation to react, close to the catalytic groups on the active enzyme complex and any other substrates. In this way the binding energy is used partially in order to reduce the contribution of the considerable activation entropy, due to the loss of the reactants’, and catalytic groups’ translational and rotational entropy, towards the total activation energy. Other contributing factors are the introduction of strain into the reactants (allowing more binding energy to be available for the transition state), provision of an alternative reactive pathway and the desolvation of reacting and catalysing ionic groups.
Enzyme-Catalyzed Reaction
The Organic Chemistry of Enzyme-Catalyzed Reactions is not a book on enzymes, but rather a book on the general mechanisms involved in chemical reactions involving enzymes. An enzyme is a protein molecule in a plant or animal that causes specific reactions without itself being permanently altered or destroyed.
Key Features
Illustrates the organic mechanism associated with each enzyme-catalyzed reaction Makes the connection between organic reaction mechanisms and enzyme mechanisms Compiles the latest information about molecular mechanisms of enzyme reactions Accompanied by clearly drawn structures, schemes, and figures Includes an extensive bibliography on enzyme mechanisms covering the last 30 years Explains how enzymes can accelerate the rates of chemical reactions with high specificity Provides approaches to the design of inhibitors of enzyme-catalyzed reactions Categorizes the cofactors that are appropriate for catalyzing different classes of reactions Shows how chemical enzyme models are used for mechanistic studies Describes catalytic antibody design and mechanism Includes problem sets and solutions for each chapter Written in an informal and didactic style.
Enzyme Diffusivity
The advent of single-molecule studies led in the 2010s to the observation that the movement of untethered enzymes increases with increasing substrate concentration and increasing reaction enthalpy. Subsequent observations suggest that this increase in diffusivity is driven by transient displacement of the enzyme’s center of mass, resulting in a “recoil effect that propels the enzyme”.