An acid anhydride is a compound with two acyl groups bound to a single oxygen atom. It is a type of chemical compound derived by the removal of water molecules from an acid. It is a nonmetal oxide which reacts with water to form an acidic solution. It also refers to compounds containing the acid anhydride functional group. The most common anhydrides in organic chemistry are those derived from carboxylic acids. They are the molecules that are capable of forming acidic solutions in water.
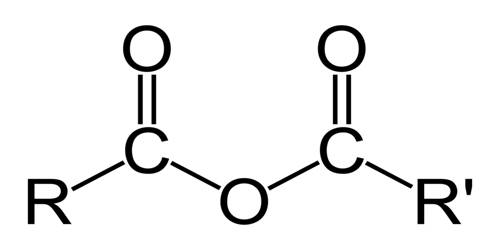
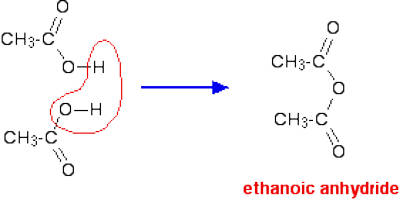
In organic chemistry, organic acid anhydrides contain the functional group R(CO)O(CO)R’. It is a functional group consisting of two acyl groups joined together by an oxygen atom. Organic acid anhydrides often form when one equivalent of water is removed from two equivalents of an organic acid in a dehydration reaction.
- Acids – These are the substances that are ready to donate hydrogen ions in water.
- Bases – these are the substances that hydroxide ions in water.
In inorganic chemistry, an acid anhydride refers to an acidic oxide, an oxide that reacts with water to form an oxyacid (an inorganic acid that contains oxygen or carbonic acid), or with a base to form a salt. So, it is a compound formed by removing water from a more complex compound: an oxide of a nonmetal (acid anhydride) or a metal (basic anhydride) that forms acid or a base, respectively, when united with water.
Reactions:
Formation of Carbonic acid – When carbon dioxide reacts with water, it forms sulphuric acid. The chemical equation will be like this. CO2(g) + H2O → H2CO3(aq)
Formation of Sulphuric acid – When Sulphur trioxide reacts with water it forms Sulphuric acid. It can be chemically explained like this. SO3(g) + H2O → H2SO4(aq)
Physical properties –
- Appearance – Ethanoic anhydride is a colorless liquid, smelling strongly of vinegar (ethanoic acid).
- Solubility in water – Ethanoic anhydride can’t be said to dissolve in water because it reacts with it to give ethanoic acid.
- Boiling point – Ethanoic anhydride boils at 140°C.
Acid anhydrides are named from the acids that created them. The O=C—O—C=O group in a carboxylic acid anhydride is called the anhydride group. The “acid” part of the name is replaced with “anhydride.” For example, the acid anhydride formed from acetic acid would be acetic anhydride.
Uses – Acids anhydrides have wide uses in organic chemistry.
- They are used in the manufacture of many things like pharmaceuticals, industrial chemicals, explosives, and perfumes.
- Synthesis of aspirin (Acetylsalicylic acid)
- Synthesis of heroin by deacetylation of morphine.