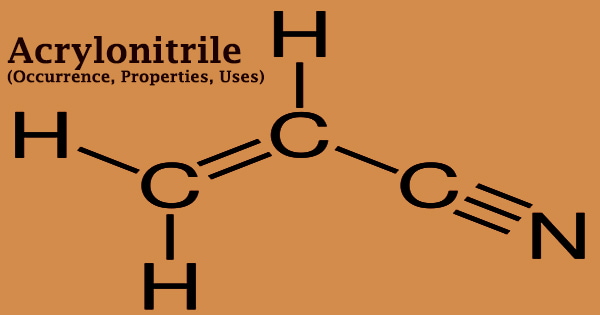
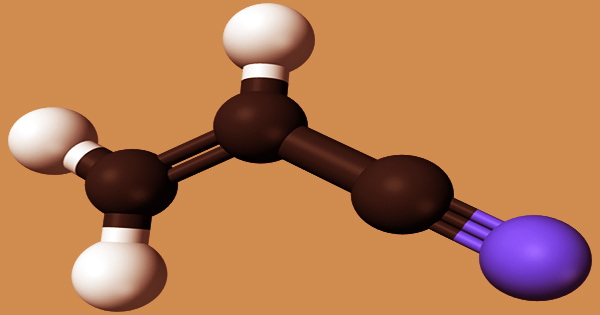
Acrylonitrile is an organic molecule with the formula CH2CHCN that is both easily volatile in air and extremely soluble in water. It’s a colorless, flammable liquid with a strong onion stench. When exposed to an open flame, its vapors may explode. Acrylonitrile is commonly used in industry to make rubber, resins, plastics, elastomers, and synthetic fibers, as well as carbon fibers for aircraft, military, and aerospace applications. Its molecular structure is made up of a vinyl group attached to a nitrile. It’s a crucial monomer in the production of useful polymers like polyacrylonitrile.
Acrylonitrile is an extensively manufactured, unsaturated nitrile that does not occur naturally. At industrial locations, however, it can reach concentrations of up to 0.11 ppm. It can linger in the atmosphere for up to a week. Other air components, such as ozone and oxygen, can also oxidize acrylonitrile. The nonbiologically mediated reaction of acrylonitrile in water is poorly understood. A headache, nausea, dizziness, poor judgment, trouble breathing, limb weakness, cyanosis, convulsions, and collapse are among symptoms of acrylonitrile exposure.
Acrylonitrile decomposes into formyl cyanide and formaldehyde when it reacts with oxygen and hydroxyl radicals. It has a negative impact on aquatic life. It’s used to produce plastics, synthetic rubber, and acrylic fibers, among other things. It was once employed as a pesticide fumigant, but all pesticide applications have since been ceased. Acrylonitrile is suspected of being a human carcinogen, and it has been linked to an elevated risk of lung and prostate cancer.
Following emissions to the air, water, soil, and groundwater, acrylonitrile exposure may occur in the environment. This chemical intermediate is widely employed in the production of medicines, antioxidants, and colors, as well as in organic synthesis. It was discovered in the atmosphere of Titan, Saturn’s moon. Computer models show that on Titan, circumstances exist that allow the chemical to form azotosomes, which are structures comparable to cell membranes and vesicles on Earth.
In the past, a pesticide made of acrylonitrile and carbon tetrachloride was utilized; however, all pesticides have been phased out. When acrylonitrile is heated between 200 and 300 °C in an inert environment, cyclization occurs, along with a slew of other processes. The SOHIO method, also known as catalytic ammoxidation of propylene, produces it. The global manufacturing capacity was projected to be 5 million tonnes per year in 2002.
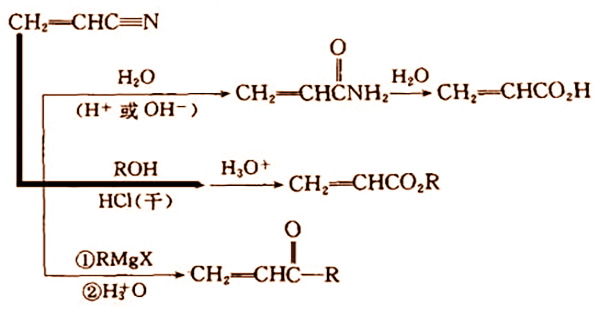
Acrylonitrile is a chemically active compound having a cyano, carbon-carbon double bond that allows it to engage in a wide range of reactions:
Polyacrylamide and polypropylene both use acrylonitrile as a basic ingredient. It can also be absorbed through contact with the skin. However, due to its ease of breakdown and disposal, it is unlikely to be kept in plants or animals. Significant byproducts such as acetonitrile and hydrogen cyanide are collected and sold. Acrylonitrile is extremely poisonous to insects, and it is the most toxic fumigant used to treat a variety of stored grain pests.
Stabilized acrylonitrile is a clear white liquid with a strong unpleasant odor. It may be used alone or in conjunction with carbon tetrachloride, and it has no impact on the germination of many vegetables, cereals, and flower seeds, although it can harm maize seeds. Excess propylene, carbon monoxide, carbon dioxide, and dinitrogen are released directly to the environment or burned if they do not dissolve. Acrylonitrile, acetonitrile, hydrocyanic acid, and ammonium sulfate make up the aqueous solution.
Vapors are more dense than air. Combustion emits hazardous nitrogen oxides, which must be stored and handled in closed systems. A recovery column eliminates the bulk water, and distillation separates the acrylonitrile and acetonitrile. The majority of stored cereal pests can be controlled with a combination of acrylonitrile and carbon tetrachloride. The results indicated that a 1:1 combination of acrylonitrile and carbon tetrachloride may be used to suppress the Phthorimaea operculella Zell found in potato tubers during storage without harming them.
Acrylonitrile is primarily utilized as a monomer in the production of polyacrylonitrile, a homopolymer, and a number of significant copolymers. It’s also utilized in office equipment, baggage, building materials, and the production of styrene-acrylonitrile (SAN) polymers for automobiles, home products, and packaging. Acrylonitrile is easily absorbed through the skin and the respiratory and gastrointestinal systems.
During World War II, Germany devised a manufacturing method that used epoxidation of ethylene, followed by hydrogen cyanide addition to generate cyanide ethanol, and lastly dehydration. Later, under the catalysis of cuprous chloride, the addition of hydrogen cyanide to acetylene was established. Adiponitrile is produced by hydrodimerizing acrylonitrile, which is utilized in the manufacture of some nylons:
2 CH2=CHCN + 2 e− + 2 H+ → NCCH2CH2CH2CH2CN
Acrylonitrile is easily polymerized, allowing for the production of polyacrylonitrile fiber (under the trade name of acrylic or bulk). It has a short fiber that resembles wool, often known as synthetic wool. It’s also used as a fumigant in small doses. In Diels–Alder processes, acrylonitrile and its derivatives, such as 2-chloroacrylonitrile, are dienophiles.
The production of carbon fibers is an emerging specialty use for acrylonitrile. These are used to strengthen composites for high-performance applications in the aviation, defense, and aerospace sectors, and are made by pyrolysis of oriented polyacrylonitrile fibers.
Information Sources: