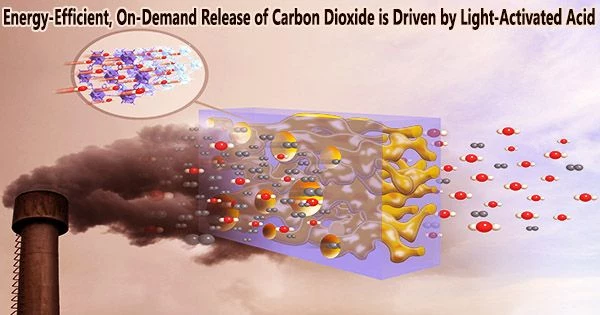
Researchers at the Department of Energy’s Oak Ridge National Laboratory have discovered a novel method to release carbon dioxide (CO2) from a solvent used in direct air capture, or DAC, to trap this greenhouse gas. Rather than using heat, this new method uses light. The innovative method opens the door to an economically viable method of removing CO2 from the atmosphere.
Because a new acid’s long-lived excited state uses ultraviolet light to control the proton concentration in the solution, it is able to release carbon dioxide on demand while also being energy-efficient.
Contrarily, modern DAC technologies pass air through an aqueous solution containing a sorbent substance, like an amino acid, that absorbs and retains ambient CO2. The solvent is heated, which releases CO2 and regenerates amino acids for recycling. The CO2 can either be stored or processed into goods of added value, such ethanol, polymers, or concrete.
“In the existing direct-air-capture technologies, CO2 release and sorbent regeneration are the most energy-intensive steps,” said ORNL chemist Yingzhong Ma, who led the study published in Angewandte Chemie International Edition with ORNL colleagues Radu Custelcean and Uvinduni Premadasa, both chemists. “The goal here is to use the amino acid sorbent, which is recyclable and has a lot of attractive properties, combined with a more energy-efficient approach to release the CO2 and regenerate the sorbent.”
The National Academy of Sciences has concluded that DAC technologies have a role in removing billions of tons of CO2 from the atmosphere annually to help limit the rise in average global temperature to less than 2 degrees Celsius (about 4 degrees Fahrenheit).
However, such a large-scale deployment is a major problem that calls for the creation of new DAC processes due to the high energy cost associated with sorbent regeneration and CO2 release at a scale that would ameliorate climate change. The ORNL-led approach provided a proof of concept for using irradiation with ultraviolet light under ambient conditions instead of heating the solution to release the CO2 and regenerate the sorbent.
“Heating aqueous solutions is a common regeneration method, but it is extremely energy intensive,” said Custelcean, a pioneer in DAC. “We wanted to take heat out of the equation.”
Custelcean led a study in 2017 that proved a guanidine sorbent could directly capture CO2 from air. In 2018, he and colleagues demonstrated a practical, energy-efficient DAC method using solar heat to drive the release of the greenhouse gas from an amino-acid sorbent. This year, Knoxville-based startup Holocene licensed the technology to prepare it for industrial deployment.
In the existing direct-air-capture technologies, CO2 release and sorbent regeneration are the most energy-intensive steps. The goal here is to use the amino acid sorbent, which is recyclable and has a lot of attractive properties, combined with a more energy-efficient approach to release the CO2 and regenerate the sorbent.
Yingzhong Ma
In this novel invention, a photoacid a molecule that gets more acidic when it absorbs light is the key to releasing CO2 under ambient conditions. Shine a light on an acid such as vinegar and nothing happens.
A chemical group in the midst of a photoacid, however, rotates from the opposing side of a bond to the same side when exposed to ultraviolet or visible light. A hydrogen ion, or proton, is transferred to the water solvent as a result of a subsequent process that creates a ring.
This transfer dramatically increases the acidity of the solution, producing a change called a “pH swing.” The excess protons can now interact with bicarbonate, or HCO3–, which was made when CO2 reacted with the sorbent. A proton is taken up by the bicarbonate to form carbonic acid, or H2CO3, which is only one step away from carbon dioxide and water in terms of energy.
“This paper describes the first time where the macroscopic pH swing lasting from minutes to hours has been demonstrated using light as an external trigger to initiate the CO2 regeneration reaction,” said Vyacheslav “Slava” Bryantsev, leader of ORNL’s Chemical Separations group and a co-author of the paper.
“You can easily turn light on and off to control the reaction reversibly,” Ma said. “You can capture CO2 in the dark and then simply turn on the light when you want to release CO2 for storage or for making value-added products. It gives you a way to easily control the process on demand.”
That said, the researchers needed an additional trick of the light. Conventional photoacids would not work because the lifetimes of their excited states are very short mere nanoseconds. They lose protons but then stay mostly in the same configuration. “Then you only change the acidity for a short time,” Bryantsev said.
Ma and Custelcean, who conceived the idea of using a photoacid to trigger CO2 release in DAC applications, ran into this problem when they began experiments using a commercially available photoacid.
“When carbonic acid decomposes, it has a short lifetime in water, on the order of a few seconds. But that’s an infinity compared to the lifetime of a regular photoacid, which is nanoseconds, or billionths of seconds,” Custelcean said. “That’s why you cannot do this chemistry with a regular photoacid: It takes seconds to release CO2 from carbonic acid, but it takes only nanoseconds for the photoacid to take the proton back.”
Bryantsev came up with the idea to try a different class of photoacid with a long-lived excited state. Called a metastable-state photoacid, it has a structure that persists in solution from seconds to hours. That means the pH change driven by the photoacid’s structural change also lasts a lot longer.
The scientists invited an expert in photoacid design and synthesis to join the team. Florida Institute of Technology’s Yi Liao had pioneered the new class of metastable-state photoacids around 2015 but for purposes other than DAC.
“We really made a breakthrough after we got this photoacid from our collaborator,” Ma said.
Custelcean agreed. “Having a metastable-state photoacid gave us plenty of time to release the proton and form the carbonic acid. Then the carbonic acid had time to release the CO2 in water. Once that happens, CO2 leaves the solution,” he said.
With Ma, first author Premadasa designed and conducted the experiments for the proof-of-concept study using a metastable-state photoacid synthesized by Liao and Florida Tech colleague Adnan Elgattar, with subsequent spectroscopic characterization by ORNL’s Benjamin Doughty and Vera Bocharova.
“Once we baselined the photochemical properties of the acid itself, our next step was to test its applicability for CO2 release with various DAC sorbents,” Premadasa said. “We can easily manipulate chemical compositions and intensities and colors of light to drive the photoreaction for efficient CO2 release.”
Audrey Miles from the University of Notre Dame and Stella Belony from the University of Florida, who were DOE Science Undergraduate Laboratory Internships students at the time of the study, tested the photoacid under different conditions for its CO2-releasing abilities. Then ORNL’s Michelle Kidder, Diana Stamberga and Joshua Damron measured the amount of CO2 released under those different conditions.
Many challenges remain to develop ORNL’s light-activated DAC technology. One is understanding the dynamics by which the photoacid forms a chemical complex with the amino acid sorbent. Another is improving the solubility of compounds in water.
Yet another is optimizing the absorption of light from the visible spectrum. The scientists also hope to gain a better grasp of the photoacid’s long-term stability and shorten the time needed to replenish it.
Regardless, the future is bright for metastable-state photoacids. “Our study paves the way towards photochemically driven approaches for CO2 release and sorbent regeneration using solar light,” Premadasa said.
The title of the paper is “Photochemically-Driven CO2 Release Using a Metastable-State Photoacid for Energy Efficient Direct Air Capture.”