Adhesion is the tendency of dissimilar particles or surfaces to cling to one another as a result of intermolecular forces. In chemistry, it refers to the tendency of some substances to cling to other substances. The most important adhesive substance on Earth is water.
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). It is the attraction of molecules of one kind for molecules of a different kind, and it can be quite strong for water, especially with other molecules bearing positive or negative charges. It is usually caused by interactions between the molecules of the two substances.
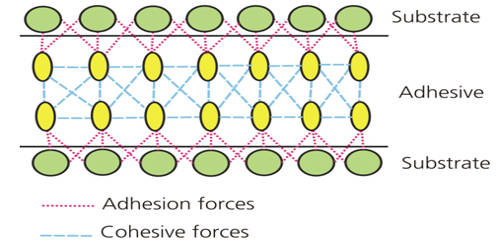
The forces that cause adhesion and cohesion can be divided into several types. The force of adhesion is defined as the force of attraction between different substances, such as glass and water. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. The term “cohesive forces” is a generic term for the collective intermolecular forces (e.g., hydrogen bonding and van der Waals forces) responsible for the bulk property of liquids resisting separation. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects.
Mechanisms
There is no single theory covering adhesion, and particular mechanisms are specific to particular material scenarios. Five mechanisms of adhesion have been proposed to explain why one material sticks to another:
- Mechanical
Adhesive materials fill the voids or pores of the surfaces and hold surfaces together by interlocking. Other interlocking phenomena are observed on different length scales. Sewing is an example of two materials forming a large scale mechanical bond, velcro forms one on a medium scale, and some textile adhesives (glue) form one at a small scale.
- Chemical
Two materials may form a compound at the joint. The strongest joints are where atoms of the two materials share or swap electrons (known respectively as covalent bonding or ionic bonding). This force tends to unite molecules of a liquid, gathering them into relatively large clusters due to the molecules’ dislike for its surroundings. A weaker bond is formed if a hydrogen atom in one molecule is attracted to an atom of nitrogen, oxygen, or fluorine in another molecule, a phenomenon called hydrogen bonding.
Strength
Adhesion is generally the force of attraction present between the water molecules and the walls of xylem vessels. The strength of the adhesion between two materials depends on which of the above mechanisms occur between the two materials, and the surface area over which the two materials contact. It is the ability of molecules/particles of a substance to stick to the molecules/particles of another substance – ie water sticking to glass surfaces. Materials that wet against each other tend to have a larger contact area than those that do not. Wetting depends on the surface energy of the materials. Capillary action and meniscus (the curved surface which is formed by any liquid in a cylinder) are the effects of adhesion.
Low surface energy materials such as polyethylene, polypropylene, polytetrafluoroethylene, and polyoxymethylene are difficult to bond without special surface preparation. A strong adhesion force causes the liquid to spread all over the surface.