Allotrope
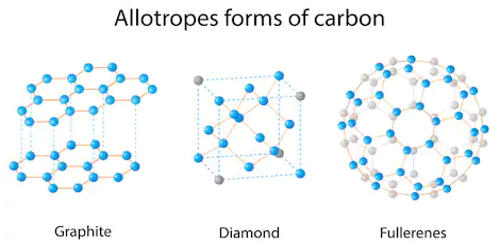
The term allotrope refers to one or more forms of a chemical element that occur in the same physical state. Elements that can have different structures (and therefore different forms), such as carbon (diamonds, graphite, and fullerene). The different forms arise from the different ways atoms may be bonded together. Elements may change allotropes in response to changes in pressure, temperature, and exposure to light. The most notable examples of allotropes are found in groups 14, 15, and 16 of the periodic table.
Allotropy is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes may display very different chemical and physical properties. For example, graphite and diamond are both allotropes of carbon that occur in the solid-state. The existence of different crystalline forms of an element is the same phenomenon that in the case of compounds is called polymorphism.
Allotropes are different structural modifications of an element; the atoms of the element are bonded together in a different manner. For example, the allotropes of carbon include diamond, graphite, graphene, and fullerenes. The term allotropy is used for elements only, not for compounds. This does not refer to the state of an element, whether solid, liquid, gas, or plasma. It refers to the tendency of an element to exist in different structural forms. The more general term, used for any crystalline material, is polymorphism. Allotropy refers only to different forms of an element within the same phase (i.e.: solid, liquid, or gas states); the differences between these states would not alone constitute examples of allotropy.
Allotrope refers to one of two or more existing forms of an element. For some elements, allotropes have different molecular formulae despite the difference in phase; for example, two allotropes of oxygen (dioxygen, O2, and ozone, O3) can both exist in the solid, liquid and gaseous states. For example, coal and graphite are different forms of carbon. The concept of allotropes was proposed by Swedish scientist Jons Jakob Berzelius in 1841.
Differences in properties of an element’s allotropes
Allotropes are different structural forms of the same element and can exhibit quite different physical properties and chemical behaviors. They differ from each other structurally depending on the number of atoms in a molecule of the element. The change between allotropic forms is triggered by the same forces that affect other structures, i.e., pressure, light, and temperature. For instance, iron changes from a body-centered cubic structure (ferrite) to a face-centered cubic structure (austenite) above 906 °C, and tin undergoes a modification known as a tin pest from a metallic form to a semiconductor form below 13.2 °C (55.8 °F). Elements exhibiting allotropy include tin, carbon, sulfur, phosphorus, and oxygen.