Allotropy (also known as allotropism) is when a chemical element can exist in two or more different forms in the same physical state or phase. It is the existence of a substance and especially an element in two or more different forms usually in the same phase. These different forms are called allotropes. The concept of allotropy was originally proposed in 1841 by the Swedish scientist Baron Jöns Jakob Berzelius (1779–1848).
Therefore, an allotrope is a different structure in which an element appears. They are different structural modifications of an element; the atoms of the element are bonded together in a different manner. This happens when the atoms of the element are bonded together in a different way. The ability for elements to exist in this way is called allotropism.
For example, the allotropes of carbon include:
- diamond, where the carbon atoms are bonded together in a four-cornered lattice arrangement;
- graphite, where the carbon atoms are bonded together in sheets of a six-sided lattice;
- graphene, single sheets of graphite; and
- fullerenes, where the carbon atoms are bonded together in spheres, cylinders or egg-shaped formations
Only some elements have allotropes.
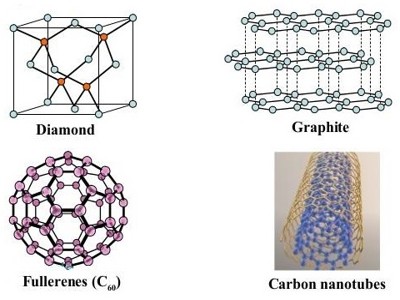
Fig: Allotropy metal structure
Allotropes may display very different chemical and physical properties. The term allotropy is used for elements only, not for compounds. The more general term, used for any crystalline material, is polymorphism. For example, graphite and diamond are both allotropes of carbon that occur in the solid-state. Allotropy refers only to different forms of an element within the same state (i.e., different solid, liquid or gas forms); these different states are not, themselves, considered examples of allotropy. Phosphorus, sulfur, and tin also exhibit allotropy. It is a property of certain elements, as carbon, sulfur, and phosphorus, of existing in two or more distinct forms; allomorphism. Many metals have allotropic crystalline forms that are stable at different temperatures.