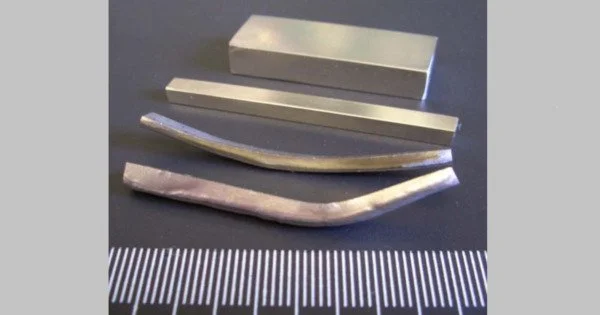
Amorphous metals, also known as metallic glasses or glassy metals, are a type of material with a disordered atomic structure, as opposed to the crystalline structure present in most metals. It is a solid metallic material, typically an alloy, having a disordered atomic-scale structure. The term “amorphous” refers to the absence of a regular, repeating atomic arrangement found in crystalline materials.
Most metals are crystalline in solid form, which means their atoms are arranged in a highly organized pattern. Amorphous metals are non-crystalline and have a glassy structure. However, unlike conventional glasses, such as window glass, which are normally electrical insulators, amorphous metals have high electrical conductivity and can exhibit metallic shine.
Amorphous metals can be generated using a variety of methods, including extremely rapid cooling, physical vapor deposition, solid-state reaction, ion irradiation, and mechanical alloying. Previously, tiny quantities of amorphous metals were made using a number of quick-cooling processes, including amorphous metal ribbons created by sputtering molten metal onto a spinning metal disk (melt spinning). The rapid cooling (millions of degrees Celsius per second) is too quick for crystals to form, leaving the material “locked” in a glassy state.
Currently, a variety of alloys with critical cooling rates low enough to allow the production of amorphous structure in thick layers (greater than 1 millimeter or 0.039 inch) have been developed; these are known as bulk metallic glasses. Recently, batches of amorphous steel with three times the strength of traditional steel alloys were produced. New techniques, such as 3D printing, which have high cooling speeds, are an important study area for bulk metallic glass fabrication.
Key characteristics of amorphous metals include:
- Structure: The atomic structure of amorphous metals lacks the long-range order seen in crystalline materials. Instead, the atoms are arranged in a more random or disordered fashion.
- Properties: Amorphous metals often exhibit unique and desirable properties, such as high strength, hardness, and corrosion resistance. They can also have good magnetic properties and excellent wear resistance.
- Processing: Amorphous metals are typically formed through rapid cooling of a molten metal alloy. This fast cooling prevents the atoms from organizing into a crystalline structure, resulting in an amorphous, glass-like material.
- Production Techniques: Common methods for producing amorphous metals include melt spinning, splat quenching, and gas atomization. These techniques allow for the rapid cooling required to achieve the amorphous structure.
Applications
Amorphous metals have found use in a variety of industries, including electronics, engineering, and biomedical equipment. They are employed in transformer cores, magnetic sensors, sports equipment, and even as coatings in some applications.
Challenges
Despite their distinct features, amorphous metals can be difficult to manufacture in large quantities due to the difficulty in managing the cooling process. Furthermore, certain amorphous alloys may be susceptible to embrittlement.
Researchers and engineers are still looking for ways to improve the characteristics and manufacturing methods of amorphous metals for use in a variety of industrial applications. The development of new alloys and manufacturing techniques may broaden the applications of these materials in a variety of industries.