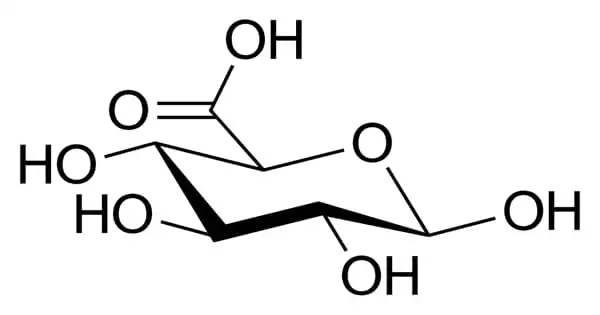
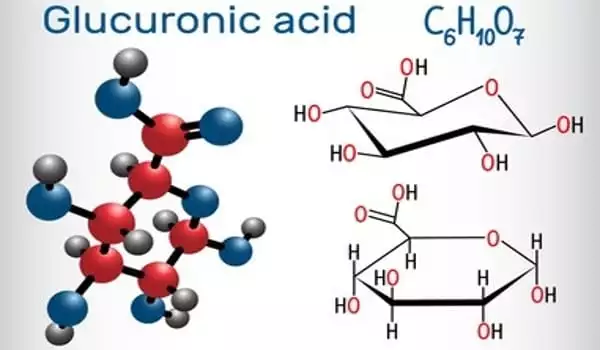
Glucuronic acid is a type of uronic acid that was discovered in urine (hence the name). The glucuronic acid pathway is a minor route of glucose metabolism in terms of volume. It is found in a variety of gums, including gum arabic (about 18%), xanthan, and Kombucha tea, and is essential for the metabolism of microorganisms, plants, and animals.
It is a type of sugar called glucose that aids in the removal of harmful substances from the body. The harmful substance and glucuronic acid combine in the liver and are then excreted in the urine. Glucuronic acid can also be found in other body tissues such as cartilage and synovial fluid (fluid found in the joints).
Properties
Glucuronic acid is a sugar acid formed by oxidizing the sixth carbon atom of glucose to form a carboxylic acid. This primary oxidation occurs in living beings with UDP-α-D-glucose (UDPG), not with free sugar.
Glucuronic acid, like its precursor glucose, can exist as a linear (carboxo-)aldohexose (<1%), or as a cyclic hemiacetal (furanose or pyranose). Aldohexoses such as D-glucose is capable of forming two furanose forms (α and β) and two pyranose forms (α and β). By the Fischer convention, glucuronic acid has two stereoisomers (enantiomers), D- and L-glucuronic acid, depending on its configuration at C-5. Most physiological sugars are of the D-configuration. Due to ring closure, cyclic sugars have another asymmetric carbon atom (C-1), resulting in two more stereoisomers, named anomers.
Epimers are carbohydrate stereoisomers that differ in configuration at only one (other) asymmetric C-atom. D-mannuronic acid (C-2), D-alluronic acid (C-3), D-galacturonic acid (C-4), and L-iduronic acid (C-5) are all epimers of glucuronic acid.
Nonplanar pyranose rings can take on a chair (in two variants) or a boat conformation. The preferred conformation is determined by spatial interference or other substituent interactions. D-glucose pyranose and its derivative D-glucuronic acid prefer chair 4C1.
Additional oxidation at C-1 to the carboxyl level yields the dicarboxylic glucaric acid. Glucuronolactone is the self-ester (lactone) of glucuronic acid.
Use
The determination of urinary steroids as well as steroid conjugates in blood. Ethyl glucuronide and ethyl sulfate are metabolites of ethanol excreted in urine and are used to monitor alcohol use or dependence. Kombucha tea fermentation products include glucuronic acid and gluconic acid.
Ascorbic acid is a precursor to glucuronic acid (vitamin C, formerly called L-hexuronic acid). Higher plants, algae, yeast, and most animals can all biosynthesize ascorbate. Every day, an adult goat produces 13 g of vitamin C. Some mammals (including humans and guinea pigs), as well as insects, invertebrates, and most fishes, lack this ability. Because they lack the biosynthetic enzyme L-gulonolactone oxidase, these species require external ascorbate supply.