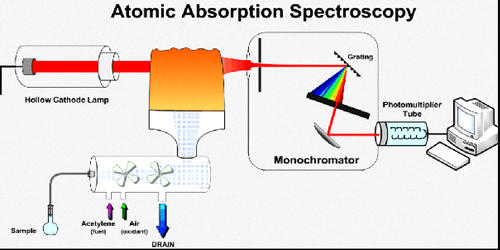
Atomic Absorption Spectroscopy (AAS) is a technique in which free gaseous atoms absorb electromagnetic radiation at a specific wavelength to produce a measurable signal. Atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state. The absorption signal is proportional to the concentration of those free absorbing atoms in the optical path. Atomic absorption spectroscopy is based on the absorption of light by free metallic ions.
In analytical chemistry, the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. Therefore, for AAS measurements the analyte must be first converted into gaseous atoms, usually by application of heat to a cell called an atomizer. AAS can be used to determine over 70 different elements in solution, or directly in solid samples via electrothermal vaporization, and is used in pharmacology, biophysics, archaeology and toxicology research.
Atomic emission spectroscopy was first used as an analytical technique, and the underlying principles were established in the second half of the 19th century by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, both professors at the University of Heidelberg, Germany. Despite these early studies, AAS was mostly restricted to astrophysical applications until the 1950s.
The modern form of AAS was largely developed during the 1950s by a team of Australian chemists. Nowadays, atomic absorption spectrometry (AAS) systems are comparatively inexpensive element selective detectors, and some of the instruments also show multi(few)-element capability. They were led by Sir Alan Walsh at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Division of Chemical Physics, in Melbourne, Australia.
Principles
The technique makes use of the atomic absorption spectrum of a sample in order to assess the concentration of specific analytes within it. The technique uses basically the principle that free atoms (gas) generated in an atomizer can absorb radiation at a specific frequency. It requires standards with known analyte content to establish the relation between the measured absorbance and the analyte concentration and relies therefore on the Beer-Lambert law.
Uses
Atomic absorption spectroscopy has become one of the most frequently used tools in analytical chemistry. Atomic absorption spectrometry has many uses in different areas of chemistry such as clinical analysis of metals in biological fluids and tissues such as whole blood, plasma, urine, saliva, brain tissue, liver, hair, muscle tissue, Atomic absorption spectrometry can use in qualitative and quantitative analysis. Atomic absorption is used for the determination of ppm levels of metals. It is not normally used for the analysis of the light elements such as H, C, N, 0, P and S, halogens, and noble gases. The electronics industry requires materials of high purity and hence there is a need to monitor trace impurity levels in materials used for electronic components.