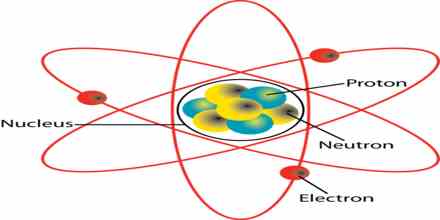
Atoms and Molecules
The atom is the smallest, indivisible particle of an element which can take part in chemical change.The molecule of an element or compound is the smallest particle of it which can normally exist separately.Most elementary gases consist of molecules containing two atoms. This state of combination of the atom is indicated by writing the molecules of these gases as H2, O2, N2, and Cl2 , meaning a single unit consisting two atoms of each the gases(2H or 2Cl would mean two separate atom of each of these gases)
Dalton‘s Atomic Theory
- Matter is made up of small, indivisible particles called atoms.
- Atoms are indestructible and cannot be created.
- The atoms of a particular element are all exactly alike in every way and are different from the atoms of all the other elements.
- Chemical combination takes place between small whole number of atoms.
Atomic Structure
After the discovery of electron n 1897, J.J. Thomson gave the following model of atomic structure.
Thomson’s Atom Model
According to his model the negatively charged electrons are embedded in a positively charged sphere just like the plums in pudding.This model hold sway until earnest Rutherford announces the nuclear model of atom in 1911.
Rutherford’s Atom Model
Following the research work of Geiger and Marsden, two research fellow working under Earnest Rutherford, it was concluded that almost of all the spaces inside atom is void with a heavy positively charged particle at the center.
This positively charged particle at the center is called the nucleus an accounts for almost all the mass of the atom. The negatively charged electron which are negligible in their size and mass rotates in circular orbits around the nucleus. The number of electrons are such as to compensate the positive charge of the nucleus so that the atom as a whole is electrically neutral.
Bohr’s model and arrangement of electron in atom
- The electrons in the atom are grouped in different shells having different energy. These are also called energy level. The shells are numbered as 1, 2, 3, 4 and so on. All electrons in a particular shell have approximately same energy.
- The maximum number of electron in the nth shell is 2n2.
- The number of electrons in the outermost shell is 8.
Structure of atoms
The lightest element hydrogen has one proton in their nucleus and one electron orbiting round it. The nest heavier element is helium having two protons and two neutrons in the nucleus and two electrons orbiting round the nucleus. The electrons of hydrogen and the helium atoms stay in the 1st shell. In the next element lithium having atomic number three and atomic mass of 6 cannot accommodate the third electron in the 1st shell and the third electron goes to the next shell corresponding to n =2. the second shell can accommodate up to 8 electrons.
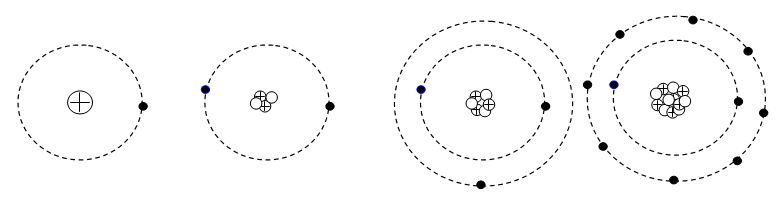
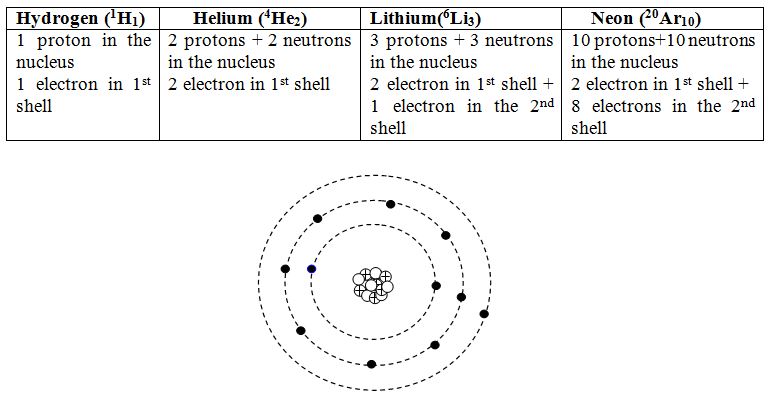
Sodium has an atomic number of 11 and atomic mass of 23. so it has 11 proton and 12 neutron in the nucleus and 11 electron in the shells. The first two shells can accommodate 10 electrons and the 11th electron goes the 3rd shell
Subatomic Particles
Although many subatomic particles are known, for most chemical purposes the atom may be considered to be composed of three particles.
- ELECTRON : The electron has single negative charge(-1.6´ 10-19 C) and a mass of 1/1836 a.m.u (atomic mass unit)
- PROTON : The proton has a single positive charge (+1.6´ 10-19 C) and a mass of 1 a.m.u.
- NEUTRON : The neutron has a no charge (zero) and a mass of 1 a.m.u.
An element is identified by the following parameters
Atomic Numbers:
The number of protons in the nucleus is the atomic number of an element. This is also called the proton number and this is the parameter which determines the chemical and the physical properties of an element.
Atomic Weight/Mass:
Atomic Mass is the relative mass of an isotope of an element with respect to the mass a hydrogen atom or one twelfth of the mass of carbon-12. The atomic mass of an element is equal to the total no of proton and neutrons in its nucleus.
It is also a characteristic property of a stable atom. However there are exceptions where same element has different Atomic mass i.e. the number of neutrons are different. This atoms having different mass number having same atomic numbers are called Isotopes of an element. Such as normally hydrogen has one proton with no neutron in its nucleus. However there are two variety of hydrogen atom which has one and two neutrons respectively in their nucleus. They are called deuterium and tritium respectively
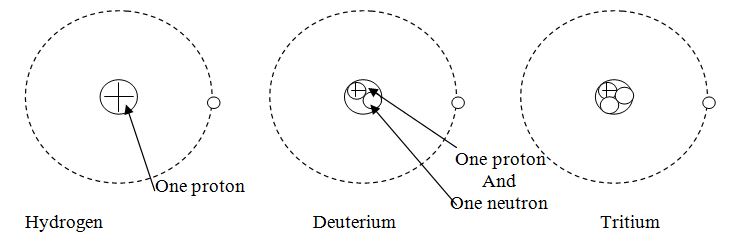
Notation for isotopes
Isotopes (nuclides) are specified by the name of the particular element by a hyphen and the number of nucleons (protons and neutrons) in the atomic nucleus (e.g., helium-3, carbon-12, carbon-14, iodine-131, uranium-238).
In symbolic form, the number of nucleons is denoted as a superscripted prefix to the chemical symbol. Such as the isotope of any element X of atomic number Z and mass number A is written as X. For example the symbols of isotopes of the above mentioned elements are He, C, C, I, U respectively
This is a list of chemical elements, sorted by atomic mass color coded according to type of element. Each element’s atomic number, name, element symbol, and group and period numbers on the periodic table are given.
Atomic Number | Name | Element Symbol | Atomic Weight | Group | Period |
1 | Hydrogen | H | 1 | 1 | 1 |
2 | Helium | He | 4 | 18 | 1 |
3 | Lithium | Li | 7 | 1 | 2 |
4 | Beryllium | Be | 9 | 2 | 2 |
5 | Boron | B | 11 | 13 | 2 |
6 | Carbon | C | 12 | 14 | 2 |
7 | Nitrogen | N | 14 | 15 | 2 |
8 | Oxygen | O | 16 | 16 | 2 |
9 | Fluorine | F | 19 | 17 | 2 |
10 | Neon | Ne | 20 | 18 | 2 |
11 | Sodium (Natrium) | Na | 23 | 1 | 3 |
12 | Magnesium | Mg | 24 | 2 | 3 |
13 | Aluminium (Aluminum) | Al | 27 | 13 | 3 |
14 | Silicon | Si | 28 | 14 | 3 |
15 | Phosphorus | P | 31 | 15 | 3 |
16 | Sulfur | S | 32 | 16 | 3 |
17 | Chlorine | Cl | 35 | 17 | 3 |
18 | Argon | Ar | 40 | 18 | 3 |
19 | Potassium (Kalium) | K | 40 | 1 | 4 |
20 | Calcium | Ca | 40 | 2 | 4 |
26 | Iron (Ferrum) | Fe | 56 | 8 | 4 |
29 | Copper (Cuprum) | Cu | 64 | 11 | 4 |
32 | Germanium | Ge | 73 | 14 | 4 |
47 | Silver (Argentum) | Ag | 108 | 11 | 5 |
48 | Cadmium | Cd | 112 | 12 | 5 |
49 | Indium | In | 115 | 13 | 5 |
50 | Tin (Stannum) | Sn | 119 | 14 | 5 |
51 | Antimony (Stibium) | Sb | 122 | 15 | 5 |
52 | Tellurium | Te | 128 | 16 | 5 |
53 | Iodine | I | 127 | 17 | 5 |
54 | Xenon | Xe | 131 | 18 | 5 |
55 | Caesium (Cesium) | Cs | 133 | 1 | 6 |
56 | Barium | Ba | 137 | 2 | 6 |
74 | Tungsten (Wolfram) | W | 184 | 6 | 6 |
78 | Platinum | Pt | 195.084(9) | 10 | 6 |
79 | Gold (Aurum) | Au | 196.966569(4) | 11 | 6 |
80 | Mercury (Hydrargyrum) | Hg | 200.59(2) | 12 | 6 |
82 | Lead (Plumbum) | Pb | 207.2(1)2 4 | 14 | 6 |
86 | Radon | Rn | [220]1 | 18 | 6 |
92 | Uranium | U | 238.02891(3)1 2 3 | n/a | 7 |
Notation for isotopes
Isotopes (nuclides) are specified by the name of the particular element by a hyphen and the number of nucleons (protons and neutrons) in the atomic nucleus (e.g., helium-3, carbon-12, carbon-14, iodine-131, uranium-238).
In symbolic form, the number of nucleons is denoted as a superscripted prefix to the chemical symbol. Such as the isotope of any element X of atomic number Z and mass number A is written as X. For example the symbols of isotopes of theabove mentioned elements are He, C, C, I, U respectively
Questions
Notation for isotopes
Isotopes (nuclides) are specified by the name of the particular element by a hyphen and the number of nucleons (protons and neutrons) in the atomic nucleus (e.g., helium-3, carbon-12, carbon-14, iodine-131, uranium-238).
In symbolic form, the number of nucleons is denoted as a superscripted prefix to the chemical symbol. Such as the isotope of any element X of atomic number Z and mass number A is written as X. For example the symbols of isotopes of the above mentioned elements are He, C, C, I, U respectively.
Questions: Copy and complete the following table of atomic structure of some elements-
| Element | Atomic number | Relative atomic mass | Number of protons | Number of neutrons | Number of electrons |
| Helium | 2 | 4 | |||
| Lithium | 7 | 3 | |||
| Boron | 11 | 6 | |||
| Carbon | 6 | 12 | |||
| Nitrogen | 14 | 7 | |||
| Oxygen | 16 | 8 | |||
| Sodium | 11 | 23 | |||
| Iron | 26 | 56 | |||
| Chlorine | 35 | 17 | |||
| Silver | 108 | 47 | |||
| Gold | 79 | 197 | |||
| Mercury | 200 | 80 | |||
| Uranium | 92 | 238 |