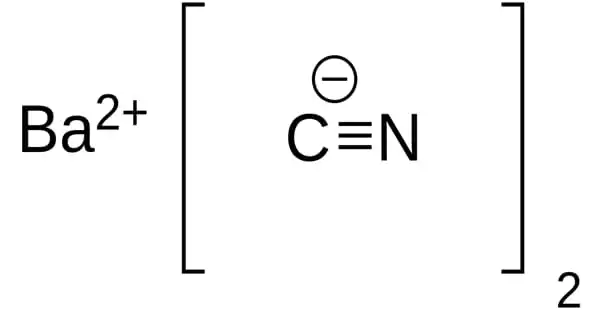
Barium cyanide is a chemical compound with the formula Ba(CN)2. It appears as a white crystalline solid. It is synthesized by the reaction of hydrogen cyanide and barium hydroxide in water or petroleum ether. It is a white crystalline salt.
It is soluble in water. Gradually decomposed by water and rapidly decomposed by acids to give off hydrogen cyanide, a flammable poison gas. It is inorganic cyanide. Members of this class that contain heavy metals tend to explosive instability, most of them are capable of violent oxidation under certain conditions; a fusion of metal cyanides with metal chlorates, perchlorates, nitrates, or nitrites can cause violent explosion
Properties
- Molecular Weight: 189.36
- Appearance: White crystalline powder
- Melting Point: 600 °C
- Boiling Point: 42.5°C
- Density: N/A
- Solubility in H2O: 18 g/100 mL (14 °C)
Reactions
Barium cyanide is a chemical compound of barium and cyanide. Barium is a metallic alkaline earth metal with the symbol Ba, and atomic number 56. It never occurs in nature in its pure form due to its reactivity with air but combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds that may be found as minerals.
Barium cyanide reacts with water and carbon dioxide in the air slowly, producing highly toxic hydrogen cyanide gas.
When barium cyanide is heated to 300° C with the steam present, the nitrogen evolves to ammonia, leaving barium formate. The reaction is Ba(CN)2 + 4H2O = Ba(HCOO)2 + 2NH3.
Aqueous solutions of barium cyanide dissolve insoluble cyanides of some of the heavy metals forming crystalline double salts. For example, BaHg(CN)4.3H2O in needles, 2Ba(CN)2.3Hg(CN)2.23H2O in transparent octahedra, and Ba(CN)2.Hg(CN)2.HgI2.6H2O.
Preparation
Barium cyanide is synthesized by the reaction of hydrogen cyanide and barium hydroxide in water or petroleum ether. This white crystalline salt reacts with water and carbon dioxide in the air slowly, producing highly toxic hydrogen cyanide gas
Barium cyanide is prepared by reacting barium hydroxide with hydrocyanic acid:
Ba(OH)2 + 2HCN → Ba(CN)2 +2H2O
The product is crystallized from the solution.
Toxicity
Toxic by skin absorption through open wounds, by ingestion, and by inhalation of hydrogen cyanide from decomposition. Toxic oxides of nitrogen are produced in fires involving this material.
Exposure to cyanides can cause headaches, vertigo, nausea, and vomiting may occur at low concentrations. High concentration causes difficult breathing, palpitation, paralysis, unconsciousness, respiratory arrest, cyanosis, and death.