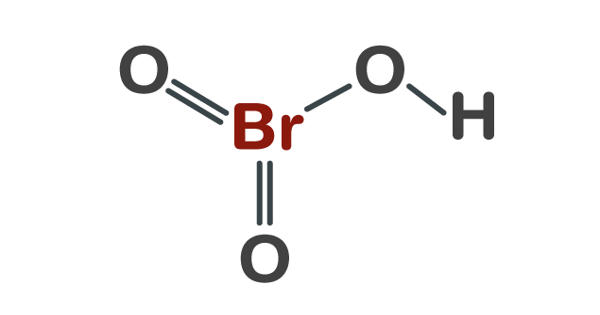
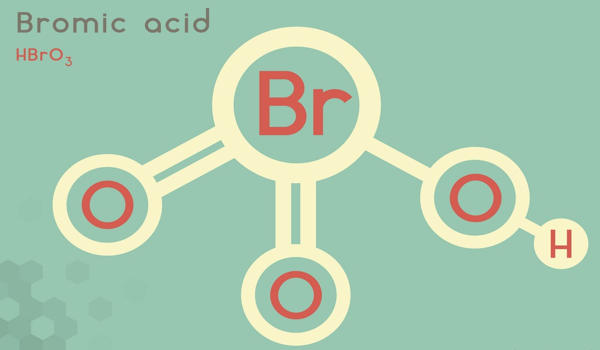
Bromic acid, also known as hydrogen bromate, is an oxoacid with the molecular formula HBrO3. It is a bromine oxoacid. It only exists in an aqueous solution. It is the conjugate acid of bromate. It is a colorless solution that turns yellow at room temperature as it decomposes to bromine. It is an oxoacid and exists only in an aqueous solution. It is a solution that is colorless.
Bromic acid and bromates are powerful oxidizing agents and are common ingredients in Belousov–Zhabotinsky reactions. Hydrogen bromate is a powerful oxidizing agent and is a common ingredient in the Belousov-Zhabotinsky reaction. Belousov-Zhabotinsky reactions are a classic example of non-equilibrium thermodynamics.
Dissociation
Low concentrations dissociate completely to hydronium and bromate while high concentrations decompose to form bromine. During its decomposition to bromine at room temperature, the colour of the solution changes yellow. Bromic acid’s high instability can be explained because the positively charged hypervalent bromine is connected to the electronegative OH group.
Properties
Bromic acid is obtained as a product in the reaction of barium bromate (Ba(BrO3)2) and sulfuric acid (H2SO4). Barium sulfate obtained is a precipitate form. Therefore bromic acid can be decanted by removing the precipitated barium sulfate.
- Molecular weight: 128.91 g/mol
- Conjugate base: Bromate
- pKa: −2
- Complexity: 46.2
Preparation
Bromic acid is prepared by adding sulfuric acid to barium bromate.
Ba(BrO3)2 + H2SO4 → 2HBrO3 + BaSO4
The product is distilled and absorbed in water. A 50% solution may be obtained by slow evaporation of the dilute solution in vacuum at -12°C.
Synthesis
The solution of bromic acid decomposes in the air upon standing and particularly upon heating. One can obtain Bromic acid through the decomposition of its barium salt with sulfuric acid. Bromic acid is the product of a reaction of barium bromate and sulfuric acid.
Ba(BrO3)2 + H2SO4 → 2 HBrO3 + BaSO4
Barium sulfate is insoluble in water and forms a precipitate. The aqueous bromic acid can be decanted removing the barium sulfate. Bromates are obtained by electrochemical oxidation of the corresponding bromides.
Uses
Bromic acid is useful for making bromates. It is an acid, stable only in very dilute solutions, usually produced by the reaction of barium bromate with sulfuric acid: used chiefly as an oxidizing agent in the manufacture of dyes and pharmaceuticals. Bromine works as a powerful oxidizing agent and is able to release oxygen-free radicals from the water in mucous membranes.
Information Source: