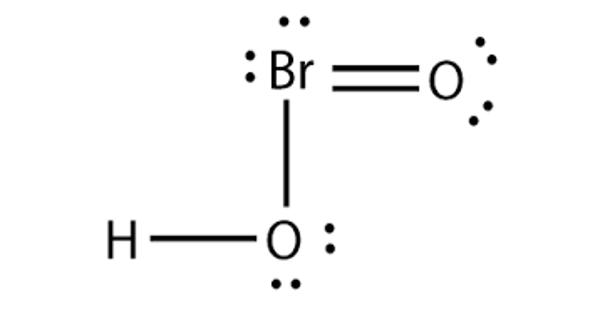
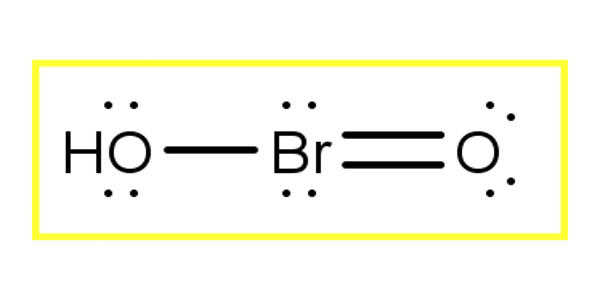
Bromous acid is the inorganic compound with the formula of HBrO2. It contains hydrogen and bromite ions. It has bromine in the +3 oxidation state. The salts of bromous acid are called bromites. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. The acid is not stable and only occurs as an intermediate; for example, in the oxidation of hypobromites. In an acidic solution, bromites decompose to bromine.
Discovery
In 1905, Richards A. H. proved the existence of bromous acid through a series of experiments involving silver nitrate (AgNO3) and bromine. The reaction of excess cold aqueous to form hypobromous acid (HBrO), silver bromide (AgBr) and nitric acid (HNO3):
Br2 + AgNO3 + H2O → HBrO + AgBr + HNO3
Richards discovered that the effect of adding excess liquid bromine in a concentrated silver nitrate (AgNO3) resulted in a different reaction mechanism.
According to Richards, hypobromous acid (HBrO) arises by the reaction of bromine and silver nitrate solution:
Br2 + AgNO3 + H2O → HBrO + AgBr + HNO3
2 AgNO3 + HBrO + Br2 + H2O → HBrO2 + 2 AgBr + 2 HNO3
Synthesis
It can be made by oxidizing hypobromous acid with hypochlorous acid. It can also be made by careful disproportionation of hypobromous acid. A oxidation reaction between hypobromous acid (HBrO) and hypochlorous acid (HClO) can be used to produce bromous acid (HBrO2) and hydrochloric acid (HCl).
HBrO + HClO → HBrO2 + HCl
A redox reaction of hypobromous acid (HBrO) can form bromous acid (HBrO2) as its product:
HBrO + H2O − 2e– → HBrO2 + 2H+
The disproportionation reaction of two equivalents hypobromous acid (HBrO) results in the formation of both bromous acid (HBrO2) and hydrobromic acid (HBr):
2 HBrO → HBrO2 + HBr
A rearrangement reaction, which results from the syn-proportion of bromic acid (HBrO3) and hydrobromic acid (HBr) gives bromous acid (HBrO2):
2 HBrO3 + HBr → 3 HBrO2
Reactivity
In comparison to other oxygen-centered oxidants (hypohalites, anions of peroxides) and in line with its low basicity, bromite is a rather weak nucleophile. It can also be made by reacting with hypobromous acid and bromic acid. They can be used to reduce permanganates to manganates. Rate constants of bromite towards carbocations and acceptor-substituted olefins are by 1–3 orders of magnitude lower than the ones measured with hypobromite.
Information Source: