Butane is a hydrocarbon and a highly flammable, colourless, odourless, easily liquefied gas. It is an alkane with the formula C4H10. It is typically used as fuel for cigarette lighters and portable stoves, a propellant in aerosols, a heating fuel, a refrigerant, and in the manufacture of a wide range of products. It is used as a fuel, an aerosol propellant, in cigarette lighters, and to make other chemicals.
Butane is a gas at room temperature and atmospheric pressure. It is shipped as a liquefied gas under its vapor pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. It contact with the liquid can cause frostbite. It is regarded as one of the more harmful volatile substances to inhale. It is easily ignited. High levels of use within a short period of time can lead to depressed breathing and loss of consciousness.
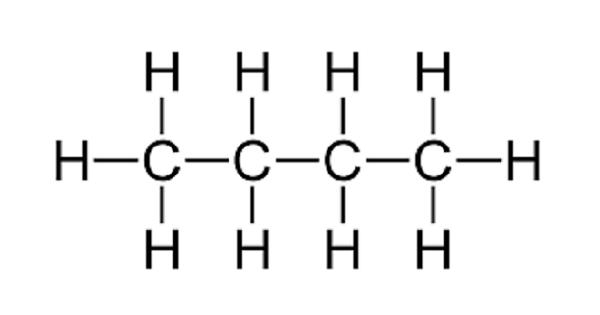
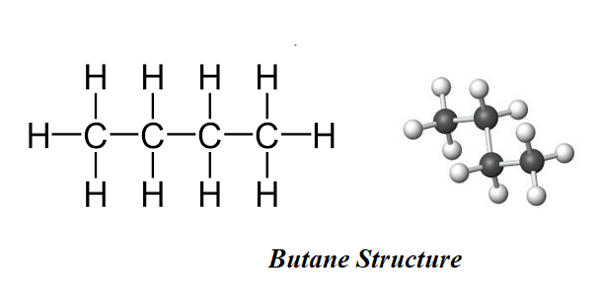
Butane is an organic compound with the formula C4H10. Butane is a saturated hydrocarbon containing 4 carbons, with unbranched structure.
It was discovered by the chemist Edward Frankland in 1849. It was found dissolved in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties.
Properties
- Density: 2.48 kg/m³
- Molecular Weight/ Molar Mass: 58.12 g/mol
- Boiling Point: -1 °C
- Melting Point: -138 °C
- Appearance: Colourless gas
- Solubility: Insoluble in water
Reactions
Butane is a straight chain alkane composed of 4 carbon atoms. When oxygen is plentiful, butane burns to form carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide may also be formed. It is a gas molecular entity and an alkane. Butane is denser than air. Its vapors are heavier than air.
When there is sufficient oxygen:
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
When oxygen is limited:
2 C4H10 + 9 O2 → 8 CO + 10 H2O
By weight, butane contains about 49.5 MJ/kg (13.8 kWh/kg; 22.5 MJ/lb) or by liquid volume 29.7 megajoules per liter (8.3 kWh/l; 112 MJ/U.S. gal; 107,000 Btu/U.S. gal).
The maximum adiabatic flame temperature of butane with air is 2,243 K (1,970 °C; 3,578 °F).
n-Butane is the feedstock for DuPont’s catalytic process for the preparation of maleic anhydride:
2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O
Butane is a liquefied petroleum gas that occurs naturally in crude oil and natural gas and is therefore a by-product of crude oil distillation (in refineries during cracking) and natural gas. n-Butane, like all hydrocarbons, undergoes free radical chlorination providing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of the chlorination is partially explained by the differing bond dissociation energies, 425 and 411 kJ/mol for the two types of C-H bonds.
Uses
- Normal butane can be used for gasoline blending, as a fuel gas, fragrance extraction solvent, either alone or in a mixture with propane, and as a feedstock for the manufacture of ethylene and butadiene, a key ingredient of synthetic rubber.
- It is one of the commonly used propellants in household and industrial aerosols and therefore can be found in numerous aerosol products.
- It is used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays such as deodorants.
- Butanes are also used in the manufacture of ethers and to adjust the vapor pressure of gasoline.
- Very pure forms of butane, especially isobutane, can be used as refrigerants and have largely replaced the ozone-layer-depleting halomethanes, for instance in household refrigerators and freezers.
- Butane is also used as lighter fuel for a common lighter or butane torch and is sold bottled as a fuel for cooking, barbecues and camping stoves.
Information Source: