Cadmium Sulfide is a crystalline, inorganic, yellow to orange-colored compound that when heated forms toxic fumes of cadmium oxides. With the formula CdS, it is an inorganic compound. Yellow is not immediately unfamiliar to sulfur springs in chemistry, but a good solid yellow is uncommon enough to comment on, and cadmium sulfide provides it with bells on. It occurs in nature with two distinct crystal structures, such as the rare minerals greenockite and hawleyite, but in the similarly formed zinc ores sphalerite and wurtzite, which are the main economic sources of cadmium, it is more common as an impurity substituent. When heated at 750° C in the sulfur atmosphere, the beta form converts to the alpha form.

(Cadmium Sulfide (CdS) Powder)
This basic compound (Cadmium Sulfide), blending one cadmium particle with one of sulfur, happens normally both as an unadulterated mineral and, all the more habitually, as a pollutant in zinc metals. As a compound that is anything but difficult to disconnect and cleanse, it is the chief wellspring of cadmium for every single business application. Its bright yellow color contributed to its adoption in the 18th century as a pigment for the “cadmium yellow” yellow paint. It dissolves at ordinary temperatures in dilute mineral acids when heated or concentrated (decomposes with H2S release).
Cadmium sulfide is really utilized as color in paints, plastics, materials, earthenware, and glass and is additionally utilized in sunlight based cells, smoke and radiation finders, light-transmitting diodes, and photomultipliers. It confers brilliant yellow to maroon, with solid maintenance of shading and protection from salts. It is also used in fluorescent displays, phosphors, and inks. Exposure to this material irritates the eyes, skin, and respiratory tract, causing damage to the lungs, resulting in shortness of breath, chest pain, and pulmonary edema, causing proteinuria and reduced renal function, as well as kidney damage.
Cadmium sulfide can be set up by the precipitation from dissolvable cadmium(II) salts with sulfide particles. This response has been utilized for gravimetric examination and subjective inorganic investigation. Indeed, even today, cadmium sulfide is as yet the primary wellspring of the metal, which at that point discovers its way into battery-powered nickel/cadmium ‘nicad’ batteries to help power our portable way of life. Photovoltaic and solar cells (for transforming solar energy into electrical energy), photoconductors (for xerography), thin-film transistors and diodes, rectifiers, scintillation counters, pyrotechnics, and smoke detectors are further applications of this compound.
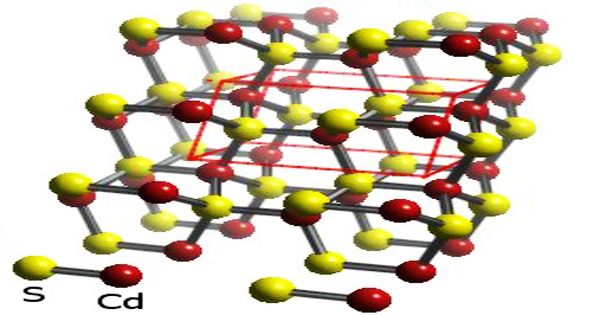
(Crystal structure of Cadmium Sulfide)
The established carcinogen calcium sulfide is associated with an elevated risk of developing lung cancer. Sulfate-reducing bacteria are also associated with it. By heating a mixture of cadmium or cadmium oxide with sulfur, the reaction between hydrogen sulfide and cadmium vapor at 800 Cor will prepare cadmium sulfide. It is possible to dissolve cadmium sulfide in acids.
CdS + 2 HCl → CdCl2 + H2S
Cadmium sulfide might be set up in shading going from white to profound orange-red relying upon the preparative technique and resultant molecule size of the material. Cadmium sulfide itself has some security issues in unvarnished artistic creations, as it can respond with air and water within the sight of bright light to deliver the dull cadmium sulfate, however, care must be taken to guarantee that the stain itself won’t cause issues. Cadmium sulfide should be kept in dark, well-ventilated areas, away from fuel and other fire hazards, and away from direct sunlight.
It may have two distinct structures when cadmium sulfide is present in the relatively uncommon pure form in nature. Cadmium sulfide can be obtained by treatment with a soluble cadmium salt in a solution of sodium or other alkali metal sulfide. The compound likewise might be set up by warming a combination of cadmium or its oxide with sulfur at 800°C; or by the response of H2S with cadmium fume at 800°C. Prior to actualizing land removal of waste cadmium sulfide, counsel natural administrative organizations for direction.
Information Sources: