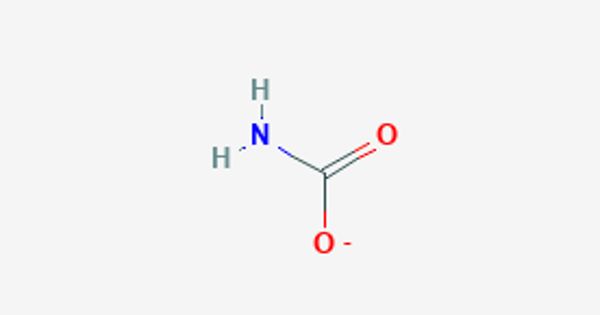
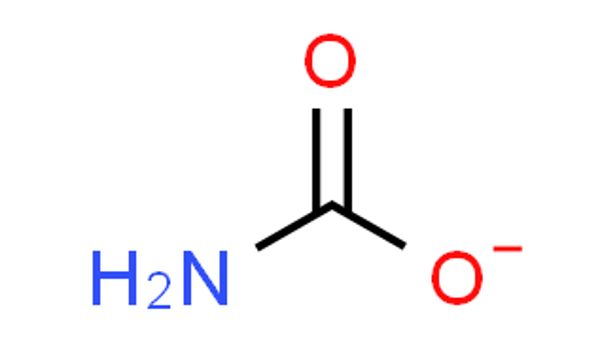
Carbamate is an amino-acid anion. It is a category of organic compounds that are formally derived from carbamic acid (NH2COOH). It is a conjugate base of carbamic acid. The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H2NCOO– (e.g. ammonium carbamate).
Polymers whose units are joined by divalent carbamate groups –NH–(C=O)–O– are an important family of plastics, the polyurethanes.
Properties
While carbamic acids are unstable, many carbamates (covalent or ionic) are stable and well known. The carbamates are a group of insecticides that includes such compounds as carbamyl, methomyl, and carbofuran.
Equilibrium with carbonate and bicarbonate
In water solutions, the carbamate anion slowly equilibrates with the ammonium NH4+ cation and the carbonate CO32- or bicarbonate HCO3– anions:
H2NCOO− + 2 H2O ↔ NH+4 + HCO−3 + HO−2 H2NCOO− + 2 H2O ↔ 2 NH+4 + 2CO2−3
Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediately, and then slowly precipitate some more as the carbamate converts.
Synthesis
Ammonium carbamate
The salt ammonium carbamate is generated by treatment of ammonia with carbon dioxide:
2 NH3 + CO2 → NH4[H2NCO2]
In general carbamate esters are referred to as urethanes, and polymers that include repeating units of carbamate are referred to as Polyurethanes.
Natural occurrence
Within nature carbon dioxide can bind with neutral amine groups to form a carbamate, this post-translational modification is known as carbamylation. This modification is known to occur on several important proteins, see examples below.
- Hemoglobin – The N-terminal amino groups of valine residues in the α- and β-chains of deoxyhemoglobin exist as carbamates. They help to stabilize the protein when it becomes deoxyhemoglobin and increases the likelihood of the release of remaining oxygen molecules bound to the protein.
- Urease and phosphotriesterase – The ε-amino groups of the lysine residues in urease and phosphotriesterase also feature carbamate. The carbamate derived from aminoimidazole is an intermediate in the biosynthesis of inosine.
Information Source: