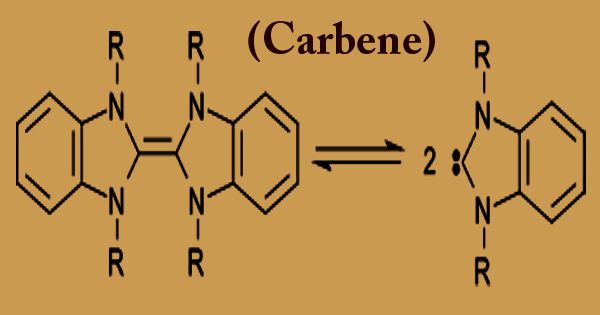
In chemistry, a carbene (H2C) any member of a class of highly reactive molecules containing divalent atoms of carbon is a carbon atom that uses only two of the four bonds they are able to form with other atoms. The general formula is R-(C:)-R ‘or R=C: where substituents or hydrogen atoms are represented by R. The four most common classes of carbon-containing organic reaction intermediates in an unstable valence state are carbenes, carbonium ions, carbanions, and free radicals. A commonplace combination including a carbene is that of cyclopropanes by the expansion of carbenes to olefins. Happening ordinarily as transient intermediates during synthetic responses, they are significant predominantly for what they uncover about compound responses and atomic structure.
The term “carbene” can also refer to a particular compound named H2C, also known as methylene, the parent hydride from which all other compounds of carbene are formally extracted. In addition, the use of carbenes can best be used to prepare certain chemical compounds, especially those in which the molecules contain carbon atoms arranged in small rings. Carbenes are delegated either singlets or trios, contingent on their electronic structure. Most carbenes are extremely brief, albeit diligent carbenes are known. Dichlorocarbene Cl2C is a well-studied carbene that can be produced in situ from chloroform and a strong base.
As indicated by the electronic hypothesis of holding, connections between molecules are framed by a sharing of electrons. As far as this hypothesis, at that point, a carbene is a compound where just two of the four valences, or holding, electrons of a carbon iota are really occupied withholding with different particles. By comparison, all four of the atoms’ valence electrons are involved in bonds with other atoms in several bonded compounds, such as hydrogen cyanide. Since the molecules of carbenes have no abundance or deficit of electrons, they are electrically neutral (nonionic).
Generally, there are two types of carbenes; singlet or triplet carbenes.
Singlet Carbene: The electrons are present with the opposite spin in various orbitals. In sp2 hybridized orbitals, the electrons are paired and comport as paired electrons.
Spin state= (2S + 1), since the electrons are antiparallel, S for singlet carbene is zero.
Therefore, Spin state = (2 × 0 + 1) = 1
Triplet Carbene: In different orbitals, all electrons are present and they hold the same spin.
Spin state = (2S + 1), since both electrons have the same spin, S for triplet carbene is 1.
Therefore, Spin state= (2 × 1 + 1) = 3
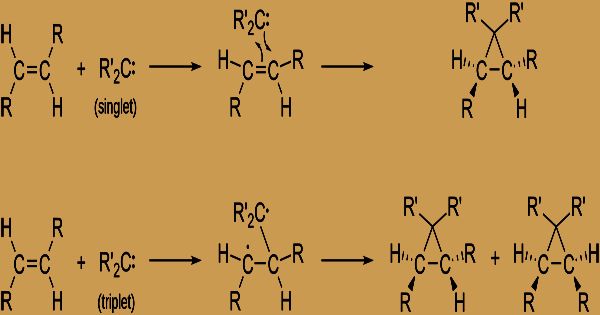
Because of the electronic spins they have, singlet and triplet carbenes are so-called. Triplet carbenes are paramagnetic, and if they can survive long enough without further reactions, they can be detected by electron spin resonance spectroscopy. The complete turn of singlet carbenes is zero while that of trio carbenes is one (in units of ħ). Bond points are 125-140° for trio methylene and 102° for singlet methylene (controlled by EPR). In a gaseous state, triplet carbenes are normally stable, whereas singlet carbenes are also found in aqueous media.
They usually have very short lifetimes due to the great reactivity of carbenes, and it is not surprising, therefore, that unambiguous and clear experimental proof of their presence has only recently been obtained. Divalent carbon mixes had been hypothesized, in any case, as quite a while in the past as 1876, when it was recommended that dichlorocarbene, Cl―C―Cl, was halfway in the base-catalyzed hydrolysis (decay achieved by water) of chloroform (HCCl3). Singlet and trio carbenes show unique reactivity. As electrophiles or nucleophiles, singlet carbenes typically participate in cheletropic reactions. Unfilled p-orbital single-carbenes should be electrophilic. It is possible to accept triplet carbenes as diradicals and engage in incremental radical additions. Triplet carbenes need to experience a moderate with two unpaired electrons while singlet carbene can respond in a solitary deliberate advance.
An extensive theory that postulated divalent carbon compounds as intermediates in several reactions was established towards the end of the 19th century. However, later studies disproved many of these postulates, and as a result, as hypothetical reaction intermediates, carbenes were no longer put forward. Carbene chemistry restored during the 1950s after unambiguous proof had shown their reality and studies by a few strategies had yielded definite data about their structures. A unique carbene’s reactivity depends on the substituent groups. Metals may influence their reactivity. Insertions into C-H bonds, skeletal rearrangements, and additions to double bonds are some of the reactions Carbenes can do. Carbenes can be delegated nucleophilic, electrophilic, or amphiphilic. Reactivity is particularly unequivocally affected by substituents. For example, if a substituent may donate a pair of electrons, a carbene is not likely to be electrophilic. There is a much more selective addition of alkyl carbenes than methylene, which does not distinguish between major, secondary, and tertiary C-H bonds.
Industrial tetrafluoroethylene processing, the precursor to Teflon, is a large-scale application of carbenes. Tetrafluoroethylene is produced through difluorocarbene intermediation:
CHClF2 → CF2 + HCl
2 CF2 → F2C = CF2
Carbenes are, in some organic reactions, short-lived intermediates. To form three-membered rings, they attack double bonds to (i.e. cyclopropane derivatives). In insertion reactions, they also target single bonds in, for example: R–O–H + R2C: → R-O-C(R2)H R–H + R2C: → R–C(R2)-H; In organic synthesis, carbenes are major ‘reagents’ and there are different techniques for producing them in the medium of reaction. On the other hand, substances with all electrons combined show no net attractive second and are alluded to as singlet states. On a basic level, carbenes can exist in either the singlet or trio state (contingent on whether the electrons are in the equivalent or diverse orbitals, separately).
Other common types of carbene reactions are insertions. Basically, the carbene interposes itself into an established bond. Usually, the order of choice is: 1. 1. Bonds with X-H where X is not carbon 2. C-H bond 3. C-C bond. Inclusions might possibly happen in a single step. Carbenes had first been hypothesized by Eduard Buchner in 1903 in cyclopropanation investigations of ethyl diazoacetate with toluene. In 1912, with diazomethane and CH2 as an intermediate, Hermann Staudinger also converted alkenes into cyclopropanes. Doering demonstrated synthetic usefulness in 1954 with dichlorocarbene.
Theoretical considerations also mean that the carbon atom and the two atoms attached to it are arranged in a “V” rather than in a linear manner, that is, in both the triplet and singlet states, the bonds from the carbon atom to the two substituent atoms are situated at an angle less than 180°. Carbenes and carbenoid forerunners can go through dimerization responses to frame alkenes. While this is frequently an undesirable side response, it very well may be utilized as an engineered apparatus and an immediate metal carbene dimerization has been utilized in the blend of polyalkynylethenes.
Since carbenes are high-energy molecules, they must be made from high-energy precursors, or they must be supplied with extra energy from external sources. Light-induced chemical transformations, so-called photochemical reactions, are also used to prepare carbenes since the energy of the light absorbed is drawn into the high-energy structures. Natural mixes containing a diazo gathering (two nitrogen molecules joined to each other and to a carbon iota by a twofold bond) are the most regularly utilized antecedents of carbenes.
Information Sources: