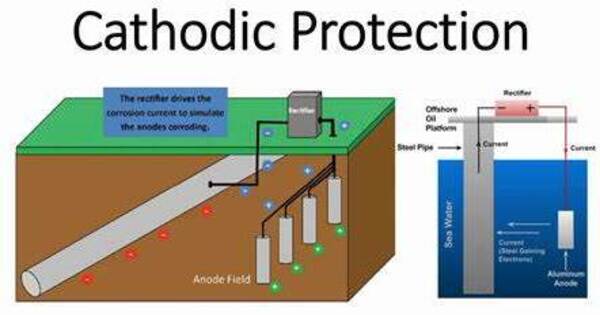
Cathodic protection is a technique for controlling corrosion on a metal surface by making it the cathode of an electrochemical cell. This is usually accomplished by placing a sacrificial anode made of a more reactive metal (such as zinc or magnesium) in contact with the metal to be protected. When these two metals are electrically linked and submerged in an electrolyte (such as dirt or water), the sacrificial anode corrodes rather than the shielded metal.
A simple technique of protection involves connecting the metal to be protected to a more readily corroded “sacrificial metal” that serves as the anode. The sacrificial metal then corrodes rather than the shielded metal. For constructions such as long pipelines where passive galvanic cathodic protection is insufficient, an external DC electrical power source is utilized to deliver sufficient current.
There are two main types of cathodic protection:
- Galvanic Cathodic Protection: This approach employs a sacrificial anode composed of a more reactive metal that corrodes preferentially to the shielded metal. The sacrificial anode is attached to the metal structure to be protected, forming a galvanic cell with the protected metal as the cathode and the sacrificial anode as the anode. Common uses include pipeline, storage tank, and ship protection.
- Impressed Current Cathodic Protection: In this procedure, an external power source, usually a rectifier, is utilized to apply an electrical current to the metal to be protected. This current is often given to a set of inert anodes spaced throughout the structure. By maintaining a constant electrical current, the shielded metal remains cathodic and does not corrode. This method is often used for large structures like pipelines and storage tanks where galvanic cathodic protection may not be sufficient.
Applications
Cathodic protection is widely used in industries such as oil and gas, marine, and utilities to prevent corrosion of metal structures, thereby extending their service life and reducing maintenance costs. Proper design, installation, and monitoring are crucial for the effective application of cathodic protection systems.
Cathodic protection systems cover a wide spectrum of metallic structures in a variety of settings. Steel water or fuel pipelines, as well as steel storage tanks like household water heaters, steel pier piles, ship and boat hulls, offshore oil platforms and onshore oil well casings, offshore wind farm foundations, and metal reinforcement bars in concrete buildings and structures, are all common applications. Another popular application is galvanized steel, which uses a sacrificial zinc coating to protect steel parts from rust.
Cathodic protection can, in some situations, stop stress corrosion cracking.